Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0794: Impact of Vaccination on Post-acute Sequelae of SARS CoV-2 Infection in Patients with Rheumatic Diseases
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Naomi Patel, MD
Massachusetts General Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Naomi Patel1, Yumeko Kawano2, Xiaosong Wang2, Xiaoqing Fu3, Claire Cook3, Kathleen Vanni2, Grace Qian2, Emily Banasiak2, Emily Kowalski2, yuqing zhang4, Jeffrey Sparks5 and Zachary Wallace3, 1Massachusetts General Hospital, Sale Creek, TN, 2Brigham and Women's Hospital, Boston, MA, 3Massachusetts General Hospital, Boston, MA, 4Massachusetts General Hospital, Quincy, MA, 5Brigham and Women's Hospital and Harvard Medical School, Boston, MA
Background/Purpose: Post-acute sequelae of COVID-19 (PASC) refers to persistent symptoms after the resolution of acute infection and is estimated to affect over 20% of COVID-19 survivors. Vaccination decreases the risk of severe acute outcomes but its impact on the etiology of PASC remains poorly understood. There are limited data on whether vaccination reduces PASC risk, especially in patients with systemic autoimmune rheumatic diseases (SARDs) where impaired responses to vaccines may leave those with breakthrough infection vulnerable to PASC.
Methods: We systematically identified SARDs patients with COVID-19 in a large multicenter academic healthcare system and invited all living patients to participate in a prospective cohort 28 days after their COVID-19 diagnosis. Patients completed surveys regarding demographics, SARD clinical characteristics, COVID-19 symptoms and their duration, and validated patient-reported outcome measures including pain, fatigue, and functional status. We defined PASC as any symptom lasting ≥ 28 days. We defined breakthrough infection as COVID-19 occurring ≥14 days after completion of the initial SARS-CoV-2 vaccine series (two doses of either the Moderna or Pfizer-BioNTech vaccines or one dose of the Johnson & Johnson-Janssen vaccine). Proportions of patients with PASC among those with and without breakthrough infection were compared using chi-square tests, and outcomes among those with PASC were compared between the two groups.
Results: Among 251 patients included in this analysis, the mean age was 53 years at COVID-19 diagnosis, 79.2% were female, and 83.3% were white (Table 1). The most common SARDs were rheumatoid arthritis (40.6%) and lupus (13.9%). The most common immunomodulatory medications were methotrexate (21.5%), TNF inhibitors (21.1%), and hydroxychloroquine (19.1%). The median (IQR) time from COVID-19 symptom onset to survey response was 191 days (94, 344). Fatigue was the most common symptom of acute COVID-19 (74.5%), followed by headache (58.6%) and fever (56.2%). The majority (86.0%) were not hospitalized. Compared to those without breakthrough infection, those with a breakthrough infection had numerically lower rates of PASC but this was not statistically significant (39% vs. 47%, p=0.2) (Figure). The most common persistent symptom was fatigue which was present in 29% of patients with PASC, followed by anosmia (18%) and dysgeusia (15%); there was a trend towards patients with breakthrough infection PASC more often having upper airway symptoms whereas non-breakthrough PASC more often had lower airway symptoms (Table 2). Pain, fatigue, and functional status scores were similar among those with PASC with and without breakthrough infection (Table 2).
Conclusion: Vaccinated patients with SARDs had numerically lower rates of PASC compared to those with no or partial prior vaccination. In addition to vaccination, our findings may also be related to differences in SARS-CoV-2 variants or acute COVID-19 treatments. Beyond reducing the severity of acute COVID-19 infection, ongoing strategies to improve vaccine uptake among SARD patients may have the additional benefit of reducing the risk of PASC.
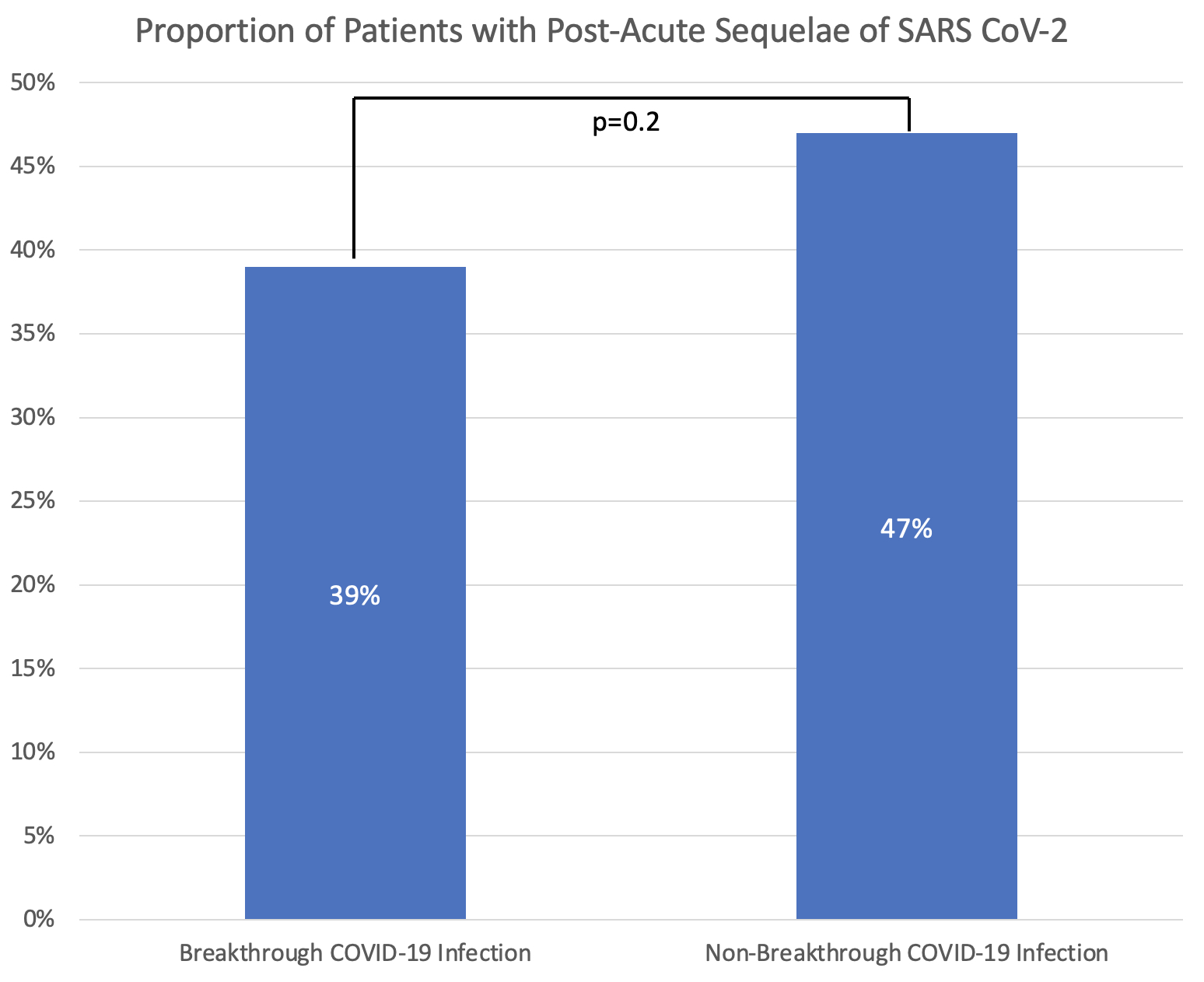 Figure. Proportion Experiencing Post-Acute Sequelae of SARS CoV-2 (PASC) among those with breakthrough and non-breakthrough COVID-19 infection
Figure. Proportion Experiencing Post-Acute Sequelae of SARS CoV-2 (PASC) among those with breakthrough and non-breakthrough COVID-19 infection
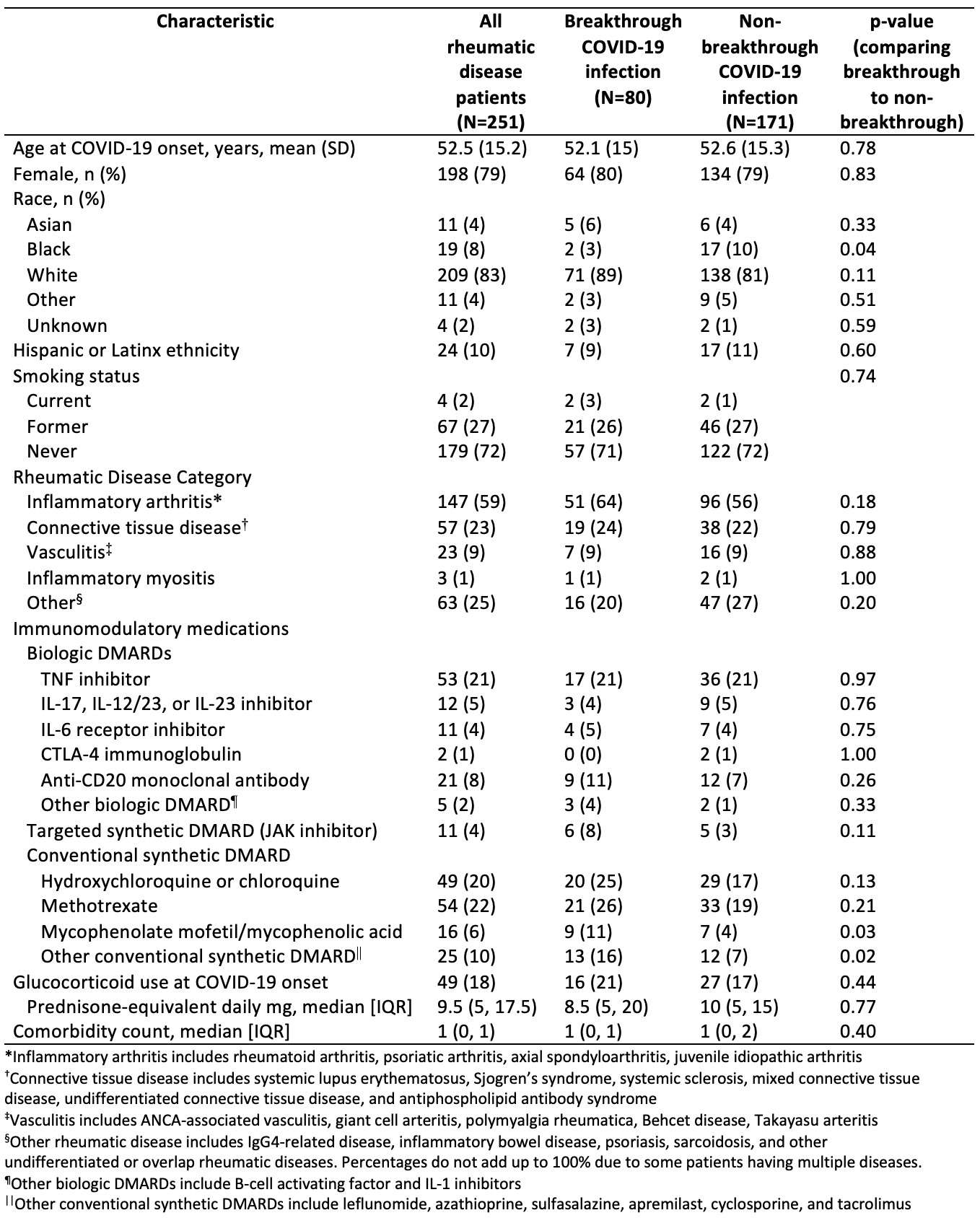 Table 1. Self-reported demographics and rheumatic disease characteristics of patients with history of COVID-19 infection
Table 1. Self-reported demographics and rheumatic disease characteristics of patients with history of COVID-19 infection
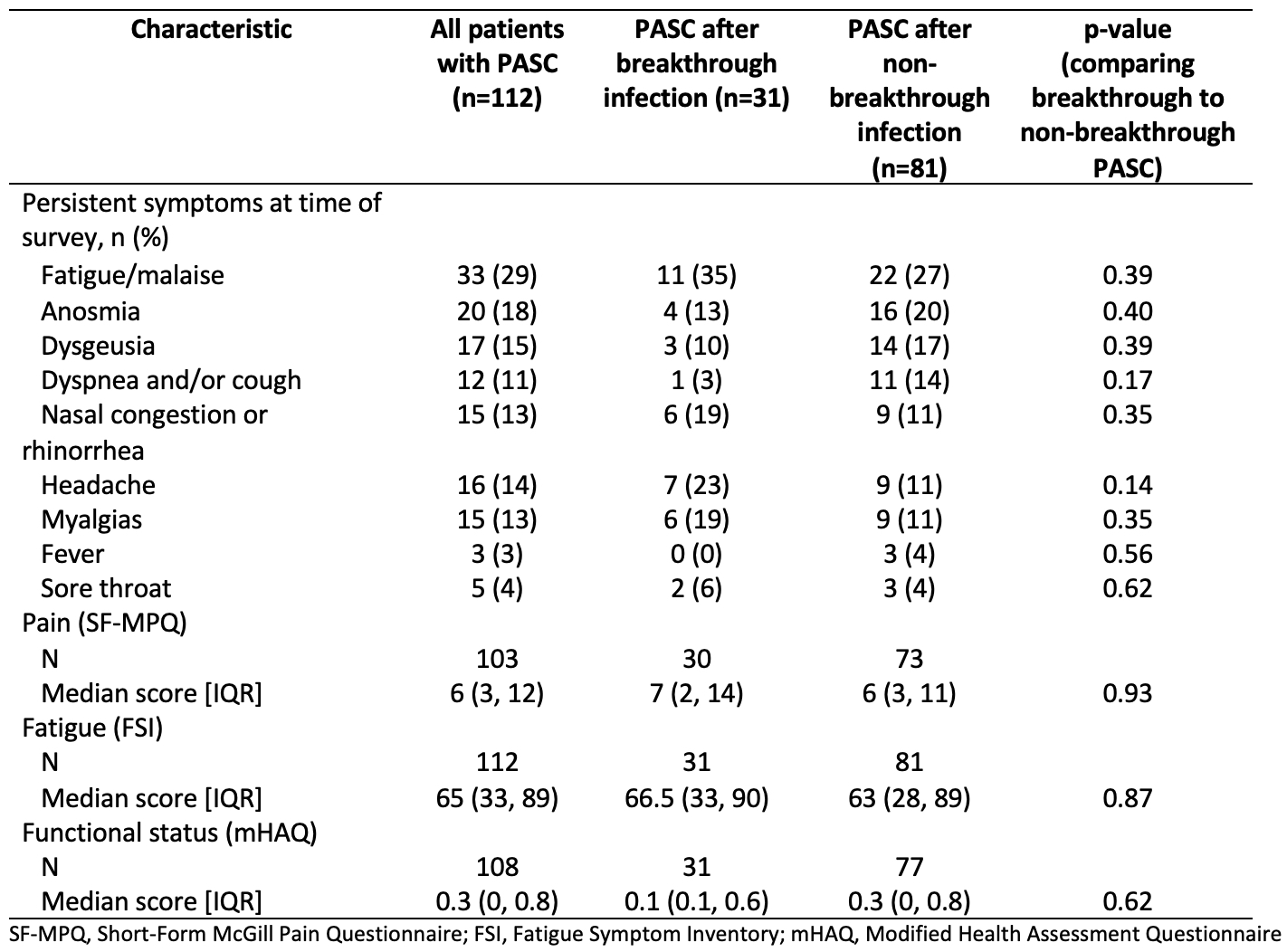 Table 2. Symptoms, pain, fatigue, and functional status scores between patients with and without breakthrough infection with PASC
Table 2. Symptoms, pain, fatigue, and functional status scores between patients with and without breakthrough infection with PASC
Disclosures: N. Patel, FVC Health; Y. Kawano, None; X. Wang, None; X. Fu, None; C. Cook, None; K. Vanni, None; G. Qian, None; E. Banasiak, None; E. Kowalski, None; y. zhang, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon.
Background/Purpose: Post-acute sequelae of COVID-19 (PASC) refers to persistent symptoms after the resolution of acute infection and is estimated to affect over 20% of COVID-19 survivors. Vaccination decreases the risk of severe acute outcomes but its impact on the etiology of PASC remains poorly understood. There are limited data on whether vaccination reduces PASC risk, especially in patients with systemic autoimmune rheumatic diseases (SARDs) where impaired responses to vaccines may leave those with breakthrough infection vulnerable to PASC.
Methods: We systematically identified SARDs patients with COVID-19 in a large multicenter academic healthcare system and invited all living patients to participate in a prospective cohort 28 days after their COVID-19 diagnosis. Patients completed surveys regarding demographics, SARD clinical characteristics, COVID-19 symptoms and their duration, and validated patient-reported outcome measures including pain, fatigue, and functional status. We defined PASC as any symptom lasting ≥ 28 days. We defined breakthrough infection as COVID-19 occurring ≥14 days after completion of the initial SARS-CoV-2 vaccine series (two doses of either the Moderna or Pfizer-BioNTech vaccines or one dose of the Johnson & Johnson-Janssen vaccine). Proportions of patients with PASC among those with and without breakthrough infection were compared using chi-square tests, and outcomes among those with PASC were compared between the two groups.
Results: Among 251 patients included in this analysis, the mean age was 53 years at COVID-19 diagnosis, 79.2% were female, and 83.3% were white (Table 1). The most common SARDs were rheumatoid arthritis (40.6%) and lupus (13.9%). The most common immunomodulatory medications were methotrexate (21.5%), TNF inhibitors (21.1%), and hydroxychloroquine (19.1%). The median (IQR) time from COVID-19 symptom onset to survey response was 191 days (94, 344). Fatigue was the most common symptom of acute COVID-19 (74.5%), followed by headache (58.6%) and fever (56.2%). The majority (86.0%) were not hospitalized. Compared to those without breakthrough infection, those with a breakthrough infection had numerically lower rates of PASC but this was not statistically significant (39% vs. 47%, p=0.2) (Figure). The most common persistent symptom was fatigue which was present in 29% of patients with PASC, followed by anosmia (18%) and dysgeusia (15%); there was a trend towards patients with breakthrough infection PASC more often having upper airway symptoms whereas non-breakthrough PASC more often had lower airway symptoms (Table 2). Pain, fatigue, and functional status scores were similar among those with PASC with and without breakthrough infection (Table 2).
Conclusion: Vaccinated patients with SARDs had numerically lower rates of PASC compared to those with no or partial prior vaccination. In addition to vaccination, our findings may also be related to differences in SARS-CoV-2 variants or acute COVID-19 treatments. Beyond reducing the severity of acute COVID-19 infection, ongoing strategies to improve vaccine uptake among SARD patients may have the additional benefit of reducing the risk of PASC.
 Figure. Proportion Experiencing Post-Acute Sequelae of SARS CoV-2 (PASC) among those with breakthrough and non-breakthrough COVID-19 infection
Figure. Proportion Experiencing Post-Acute Sequelae of SARS CoV-2 (PASC) among those with breakthrough and non-breakthrough COVID-19 infection Table 1. Self-reported demographics and rheumatic disease characteristics of patients with history of COVID-19 infection
Table 1. Self-reported demographics and rheumatic disease characteristics of patients with history of COVID-19 infection Table 2. Symptoms, pain, fatigue, and functional status scores between patients with and without breakthrough infection with PASC
Table 2. Symptoms, pain, fatigue, and functional status scores between patients with and without breakthrough infection with PASCDisclosures: N. Patel, FVC Health; Y. Kawano, None; X. Wang, None; X. Fu, None; C. Cook, None; K. Vanni, None; G. Qian, None; E. Banasiak, None; E. Kowalski, None; y. zhang, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon.

