Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0974–1003) SLE – Treatment Poster II
0986: Impact of Weight and Race on Renal Response to Cyclophosphamide in the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study (ACCESS)
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- JF
Julia Ford, MD
University of Michigan
Ann Arbor, MI, United States
Abstract Poster Presenter(s)
Julia Ford, William McCune, Neil Kamdar and Michael O'Leary, University of Michigan, Ann Arbor, MI
Background/Purpose: ACCESS assessed the efficacy of abatacept (ABA) as induction therapy in lupus nephritis (LN), randomizing 134 patients to ABA vs. placebo on a background of glucocorticoids and fixed-dose IV CYC per Euro Lupus Nephritis Trial (ELNT) [1]. While no renal response to ABA was found, the data provide an opportunity to examine predictors of renal response to fixed-dose IV CYC in a racially diverse cohort. Whether fixed-dose IV CYC is equally efficacious for larger vs. smaller patients in LN, particularly Black patients who are at risk for worse outcomes, is unknown. We performed a post-hoc analysis examining the impact of weight and race on renal response in ACCESS.
Methods: The study population was defined as participants in ACCESS excluding those who did not have a 24-hour urine protein measurement at week 24. The primary exposure was baseline weight (≥50th vs. < 50th percentile); secondary exposure was race (Black vs. non-Black). The outcome was proportion of subjects achieving urine protein < 0.8 g/day at week 24. We used multivariable logistic regression to examine the association between the interaction of weight/race with the outcome, adjusting for ABA exposure and baseline estimated glomerular filtration rate.
Results: Characteristics of the 104 patients included in our analysis are shown in Table 1. Black subjects in ≥50th percentile weight were less likely to achieve the outcome (OR 0.28, 90% CI 0.09-0.89) compared to Black subjects in < 50th percentile body weight; however, we did not observe a similar within-race effect of weight in non-Black subjects (Figure 1). In Black subjects in ≥50th percentile weight, 23.1% met the outcome, vs. 53.9% of all other subjects (p=0.01; OR 0.25 [90% CI 0.10, 0.59]).
Conclusion: In ACCESS (wherein all subjects received fixed-dose IV CYC in addition to ABA vs. placebo), Black subjects of higher weight were less likely to achieve a renal response than lighter Black patients. These findings identify Black patients of higher body weight as a high-risk group for which more aggressive dosing of IV CYC for LN (as opposed to the one-size-fits-all approach of ELNT) might be considered.
References:
1. Treatment of lupus nephritis with abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol, 2014. 66(11): p. 3096-104.
.jpeg) Except where indicated otherwise, values are the number (%). IQR= interquartile range; GFR = glomerular filtration rate; MAP = mean arterial pressure; LN = lupus nephritis.
Except where indicated otherwise, values are the number (%). IQR= interquartile range; GFR = glomerular filtration rate; MAP = mean arterial pressure; LN = lupus nephritis.
**Significant at the p < 0.10 level.
a Denotes comparison of proportion of Black subjects who did/did not meet the outcome.
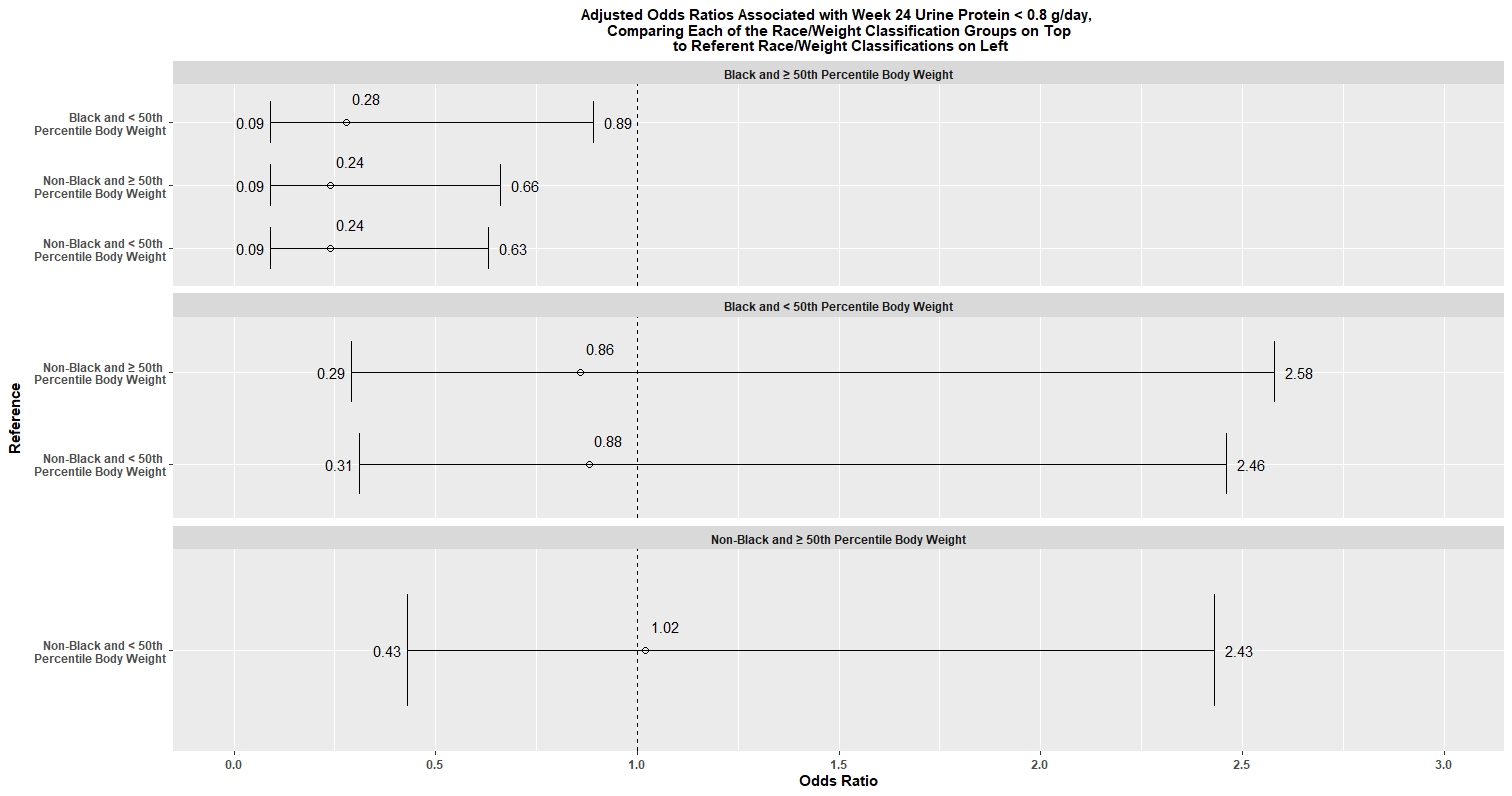 Adjusted odds ratios are reported with 90% confidence intervals.
Adjusted odds ratios are reported with 90% confidence intervals.
Disclosures: J. Ford, None; W. McCune, None; N. Kamdar, None; M. O'Leary, None.
Background/Purpose: ACCESS assessed the efficacy of abatacept (ABA) as induction therapy in lupus nephritis (LN), randomizing 134 patients to ABA vs. placebo on a background of glucocorticoids and fixed-dose IV CYC per Euro Lupus Nephritis Trial (ELNT) [1]. While no renal response to ABA was found, the data provide an opportunity to examine predictors of renal response to fixed-dose IV CYC in a racially diverse cohort. Whether fixed-dose IV CYC is equally efficacious for larger vs. smaller patients in LN, particularly Black patients who are at risk for worse outcomes, is unknown. We performed a post-hoc analysis examining the impact of weight and race on renal response in ACCESS.
Methods: The study population was defined as participants in ACCESS excluding those who did not have a 24-hour urine protein measurement at week 24. The primary exposure was baseline weight (≥50th vs. < 50th percentile); secondary exposure was race (Black vs. non-Black). The outcome was proportion of subjects achieving urine protein < 0.8 g/day at week 24. We used multivariable logistic regression to examine the association between the interaction of weight/race with the outcome, adjusting for ABA exposure and baseline estimated glomerular filtration rate.
Results: Characteristics of the 104 patients included in our analysis are shown in Table 1. Black subjects in ≥50th percentile weight were less likely to achieve the outcome (OR 0.28, 90% CI 0.09-0.89) compared to Black subjects in < 50th percentile body weight; however, we did not observe a similar within-race effect of weight in non-Black subjects (Figure 1). In Black subjects in ≥50th percentile weight, 23.1% met the outcome, vs. 53.9% of all other subjects (p=0.01; OR 0.25 [90% CI 0.10, 0.59]).
Conclusion: In ACCESS (wherein all subjects received fixed-dose IV CYC in addition to ABA vs. placebo), Black subjects of higher weight were less likely to achieve a renal response than lighter Black patients. These findings identify Black patients of higher body weight as a high-risk group for which more aggressive dosing of IV CYC for LN (as opposed to the one-size-fits-all approach of ELNT) might be considered.
References:
1. Treatment of lupus nephritis with abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol, 2014. 66(11): p. 3096-104.
.jpeg) Except where indicated otherwise, values are the number (%). IQR= interquartile range; GFR = glomerular filtration rate; MAP = mean arterial pressure; LN = lupus nephritis.
Except where indicated otherwise, values are the number (%). IQR= interquartile range; GFR = glomerular filtration rate; MAP = mean arterial pressure; LN = lupus nephritis. **Significant at the p < 0.10 level.
a Denotes comparison of proportion of Black subjects who did/did not meet the outcome.
 Adjusted odds ratios are reported with 90% confidence intervals.
Adjusted odds ratios are reported with 90% confidence intervals.Disclosures: J. Ford, None; W. McCune, None; N. Kamdar, None; M. O'Leary, None.

