Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1035–1045) Spondyloarthritis Including PsA – Treatment Poster II: Mixed
1042: Long-term Safety of Ixekizumab in Adult Patients with Psoriasis, Psoriatic Arthritis, and Axial Spondyloarthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
.png)
Atul Deodhar, MD
Professor of Medicine, Division of Arthritis and Rheumatic Diseases, School of Medicine
Oregon Health & Science University
Portland, OR, United States
Abstract Poster Presenter(s)
Atul Deodhar1, Andrew Blauvelt2, Sergio Schwartzman3, Carlo Salvarani4, Meghan Feely5, Andris Kronbergs5, Nadia Eberhart6, Danting Zhu5, Elsa Mevel5, Thorsten Holzkämper5, Eswar Krishnan7, Mark Lebwohl8, Proton Rahman9 and Helena Marzo-Ortega10, 1Oregon Health & Science University, Portland, OR, USA, Portland, OR, 2Oregon Medical Research Center, Portland, OR, USA, Portland, OR, 372nd Street Medical Associates, Scarsdale, NY, 4University of Reggio Emilia, Reggio Emilia, Italy, 5Eli Lilly and Company, Indianapolis, IN, 6Eli Lilly and Company, Vienna, Austria, 7Eli Lilly and Company, Carmel, IN, 8Icahn School of Medicine at Mount Sinai, New York, NY, 9Memorial University, St. John's, NL, Canada, 10Leeds Teaching Hospitals Trust and University of Leeds, Leeds, United Kingdom
Background/Purpose: Ixekizumab (IXE) is a high-affinity, monoclonal antibody targeting IL-17A and is approved for the treatment of psoriasis (PsO), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation. We report long-term, end-of-study-program, safety outcomes in adult patients with PsO, PsA and axSpA who received at least one dose of IXE over 5 years (PsO) or 3 years (PsA and axSpA).
Methods: An integrated safety analysis consisting of data from 25 randomised clinical trials (RCTs; 17 PsO, 4 PsA, 4 axSpA) was used to examine long-term safety of IXE. Rates of treatment-emergent adverse events (TEAEs), serious AEs (SAEs) and AEs of special interest were analyzed for all pooled studies by years of therapy and overall through March 2022, and reported as exposure-adjusted incidence rates (IRs) per 100 patient-years (PY) at successive year intervals. Additional safety outcomes included selected safety topics of interest (among others).
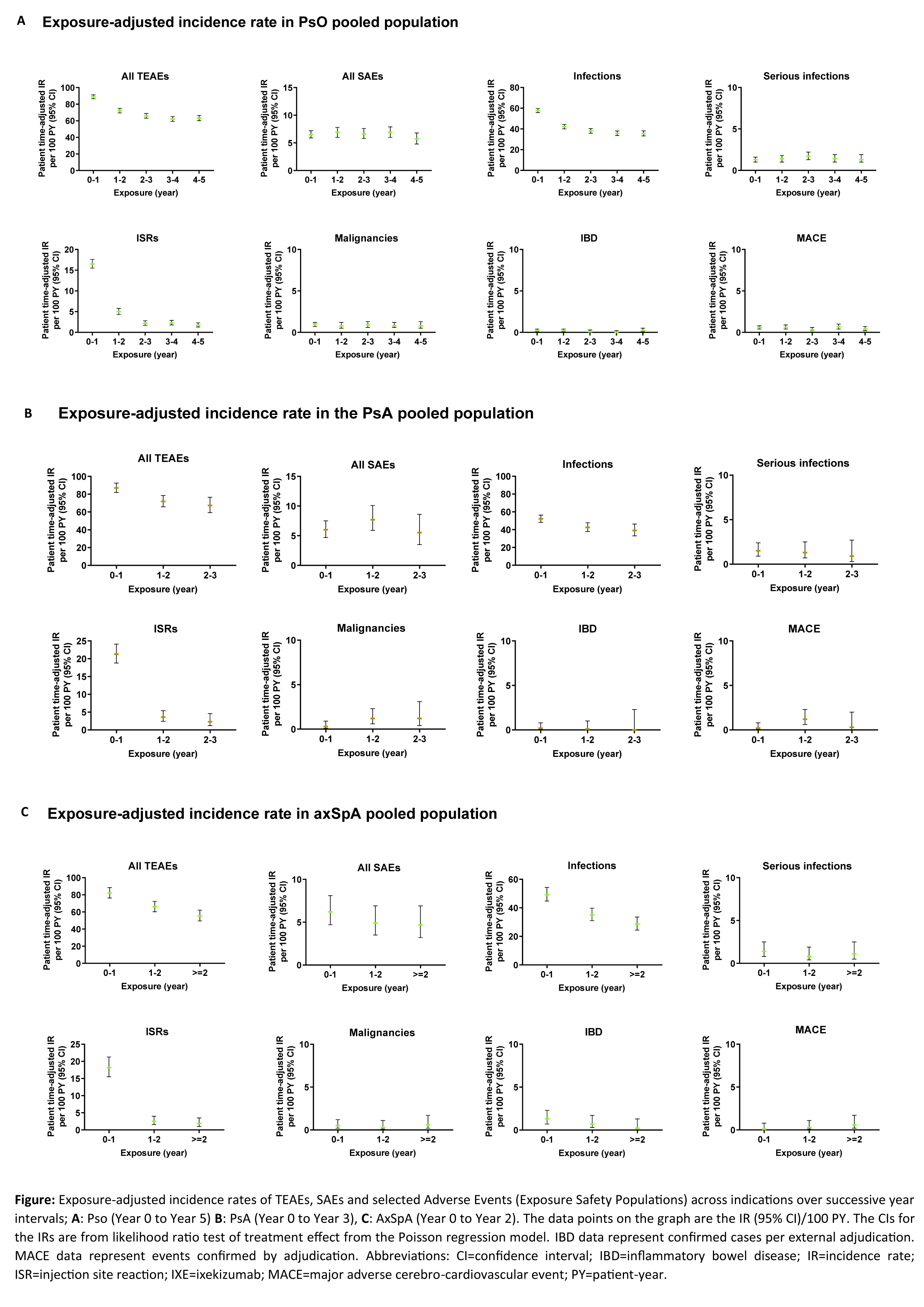
Results: A total of 6892 patients with PsO, 1401 patients with PsA, and 932 patients with axSpA, with a cumulative IXE exposure of 18025.7 PY for PsO, 2247.7 PY for PsA, and 2097.7 PY for axSpA were included in this analysis (Table, Figure). The IRs per 100 PY for any TEAE were as follows; patients with PsO=32.5, PsA=50.3, axSpA=38.0. The most commonly reported TEAEs were nasopharyngitis (PsO, IR=8.8; PsA, IR=9.0; axSpA IR=8.4) and upper respiratory tract infection (PsO, IR=6.2; PsA, IR=8.3; axSpA IR=5.8). Serious AEs were reported by 969 patients with PsO (IR=5.4), 134 patients with PsA (IR=6), and 101 patients with axSpA (IR=4.8). Forty-five deaths were reported; (PsO=36 [IR=0.2]; PsA=6 [IR= 0.3]; axSpA=3 [IR=0.1]). The IRs per 100 PY of discontinuation from the study drug due to AE were as follows: PsO, 2.9; PsA, 5.1; axSpA, 3.1. IRs of injection site reactions were: PsO, 5.9; PsA, 11.6; axSpA, 7.4. IRs of allergic reactions were: PsO, 5.6; PsA, 4.5; axSpA, 4.2. IRs of serious infections were low (PsO, IR=1.3; PsA, IR=1.2; axSpA, IR=1.1). IRs of Candida were low across all indications (PsO, 1.9; PsA, 2.0; axSpA, 1.2), as were IRs of opportunistic infections (PsO, 1.8; PsA, 1.8; axSpA, 1.3). IRs were also low across all indications for depression, major adverse cerebro-cardiovascular events and malignancies (all IRs ≤1.6, Table, Figure). Cases of inflammatory bowel disease (IBD) were uncommon (IRs ≤0.8 across indications (Table, Figure)).
Conclusion: In this updated analysis with 18025.7 PY for PsO, 2247.7 PY for PsA, and 2097.7 PY for axSpA, IXE maintained a long-term safety profile up to 5 years, consistent with previous reports1-7.
REFERENCES: 1. Mease P et al. Arthritis Care Res (Hoboken) 2019;71(3):367-78. 2. Combe B et al. Arthritis Res Ther 2020;22(1):14. 3. Genovese MC et al. Rheumatology (Oxford) 2020;59(12):3834-44. 4. Armstrong A, et al. Dermatol Ther (Heidelb). 2020;10(1):133-150. 5. Strober B, et al. J Am Acad Dermatol. 2017;76(3):432-40 e17. 6. Langley RG et al. J Eur Acad Dermatol Venereol. 2019;33(2):333-9. 7. Griffiths et al. Dermatol Ther (Heidelb). 10.1007/s13555-022-00743-9.
.jpg)
Disclosures: A. Deodhar, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer Inc, UCB Pharma, Aurinia, Moonlake; A. Blauvelt, AbbVie, Abcentra, Aligos, Almirall, Amgen, Arcutis, Arena, Aslan, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Evommune, Forte, Galderma, Incyte, Janssen, Landos, Leo Pharma, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, UCB Pharma, EcoR1, Vibliome; S. Schwartzman, AbbVie, Janssen, Eli Lilly and Company, Pfizer, Novartis, UCB, Myriad, Regeneron, Sanofi, Stelexis, Jubilant, Teijin, National Psoriasis Foundation Medical Board; C. Salvarani, None; M. Feely, Mount Sinai Hospital, NY, Eli Lilly and Company, Aerolase, Castle Biosciences, Galderma Aesthetics, Revian, Sonoma Pharmaceuticals, Suneva Medical, and Sun Pharmaceutical Industries, AAD Investment Committee, Prevention Medical Review Board, ASDS Social Media Ambassador; A. Kronbergs, Eli Lilly and Company; N. Eberhart, Eli Lilly and Company; D. Zhu, Eli Lilly and Company; E. Mevel, Eli Lilly and Company; T. Holzkämper, Eli Lilly and Company; E. Krishnan, Eli Lilly and Company; M. Lebwohl, Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Verrica, AbbVie, Amgen, Eli Lilly, Janssen, Ortho Dermatologics, Pfizer, UCB Pharma, Avotres Therapeutics, AnaptysBio, Aristea Therapeutics, BioMX, Cara Therapeutics, Castle Biosciences, Dermavant, Evommune, Facilitatation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Hexima, Meiji Seika Pharma, Mindera Health, Regeneron, Seanergy, Incyte, Arrive Technologies, Dr Reddy's Laboratories, Evelo Biosciences, Helsinn Therapeutics, LEO Pharma, Mount Sinai, CorEvitas (formerly Corrona); P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; H. Marzo-Ortega, None.
Background/Purpose: Ixekizumab (IXE) is a high-affinity, monoclonal antibody targeting IL-17A and is approved for the treatment of psoriasis (PsO), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation. We report long-term, end-of-study-program, safety outcomes in adult patients with PsO, PsA and axSpA who received at least one dose of IXE over 5 years (PsO) or 3 years (PsA and axSpA).
Methods: An integrated safety analysis consisting of data from 25 randomised clinical trials (RCTs; 17 PsO, 4 PsA, 4 axSpA) was used to examine long-term safety of IXE. Rates of treatment-emergent adverse events (TEAEs), serious AEs (SAEs) and AEs of special interest were analyzed for all pooled studies by years of therapy and overall through March 2022, and reported as exposure-adjusted incidence rates (IRs) per 100 patient-years (PY) at successive year intervals. Additional safety outcomes included selected safety topics of interest (among others).
Results: A total of 6892 patients with PsO, 1401 patients with PsA, and 932 patients with axSpA, with a cumulative IXE exposure of 18025.7 PY for PsO, 2247.7 PY for PsA, and 2097.7 PY for axSpA were included in this analysis (Table, Figure). The IRs per 100 PY for any TEAE were as follows; patients with PsO=32.5, PsA=50.3, axSpA=38.0. The most commonly reported TEAEs were nasopharyngitis (PsO, IR=8.8; PsA, IR=9.0; axSpA IR=8.4) and upper respiratory tract infection (PsO, IR=6.2; PsA, IR=8.3; axSpA IR=5.8). Serious AEs were reported by 969 patients with PsO (IR=5.4), 134 patients with PsA (IR=6), and 101 patients with axSpA (IR=4.8). Forty-five deaths were reported; (PsO=36 [IR=0.2]; PsA=6 [IR= 0.3]; axSpA=3 [IR=0.1]). The IRs per 100 PY of discontinuation from the study drug due to AE were as follows: PsO, 2.9; PsA, 5.1; axSpA, 3.1. IRs of injection site reactions were: PsO, 5.9; PsA, 11.6; axSpA, 7.4. IRs of allergic reactions were: PsO, 5.6; PsA, 4.5; axSpA, 4.2. IRs of serious infections were low (PsO, IR=1.3; PsA, IR=1.2; axSpA, IR=1.1). IRs of Candida were low across all indications (PsO, 1.9; PsA, 2.0; axSpA, 1.2), as were IRs of opportunistic infections (PsO, 1.8; PsA, 1.8; axSpA, 1.3). IRs were also low across all indications for depression, major adverse cerebro-cardiovascular events and malignancies (all IRs ≤1.6, Table, Figure). Cases of inflammatory bowel disease (IBD) were uncommon (IRs ≤0.8 across indications (Table, Figure)).
Conclusion: In this updated analysis with 18025.7 PY for PsO, 2247.7 PY for PsA, and 2097.7 PY for axSpA, IXE maintained a long-term safety profile up to 5 years, consistent with previous reports1-7.
REFERENCES: 1. Mease P et al. Arthritis Care Res (Hoboken) 2019;71(3):367-78. 2. Combe B et al. Arthritis Res Ther 2020;22(1):14. 3. Genovese MC et al. Rheumatology (Oxford) 2020;59(12):3834-44. 4. Armstrong A, et al. Dermatol Ther (Heidelb). 2020;10(1):133-150. 5. Strober B, et al. J Am Acad Dermatol. 2017;76(3):432-40 e17. 6. Langley RG et al. J Eur Acad Dermatol Venereol. 2019;33(2):333-9. 7. Griffiths et al. Dermatol Ther (Heidelb). 10.1007/s13555-022-00743-9.
.jpg)

Disclosures: A. Deodhar, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer Inc, UCB Pharma, Aurinia, Moonlake; A. Blauvelt, AbbVie, Abcentra, Aligos, Almirall, Amgen, Arcutis, Arena, Aslan, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Evommune, Forte, Galderma, Incyte, Janssen, Landos, Leo Pharma, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, UCB Pharma, EcoR1, Vibliome; S. Schwartzman, AbbVie, Janssen, Eli Lilly and Company, Pfizer, Novartis, UCB, Myriad, Regeneron, Sanofi, Stelexis, Jubilant, Teijin, National Psoriasis Foundation Medical Board; C. Salvarani, None; M. Feely, Mount Sinai Hospital, NY, Eli Lilly and Company, Aerolase, Castle Biosciences, Galderma Aesthetics, Revian, Sonoma Pharmaceuticals, Suneva Medical, and Sun Pharmaceutical Industries, AAD Investment Committee, Prevention Medical Review Board, ASDS Social Media Ambassador; A. Kronbergs, Eli Lilly and Company; N. Eberhart, Eli Lilly and Company; D. Zhu, Eli Lilly and Company; E. Mevel, Eli Lilly and Company; T. Holzkämper, Eli Lilly and Company; E. Krishnan, Eli Lilly and Company; M. Lebwohl, Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Verrica, AbbVie, Amgen, Eli Lilly, Janssen, Ortho Dermatologics, Pfizer, UCB Pharma, Avotres Therapeutics, AnaptysBio, Aristea Therapeutics, BioMX, Cara Therapeutics, Castle Biosciences, Dermavant, Evommune, Facilitatation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Hexima, Meiji Seika Pharma, Mindera Health, Regeneron, Seanergy, Incyte, Arrive Technologies, Dr Reddy's Laboratories, Evelo Biosciences, Helsinn Therapeutics, LEO Pharma, Mount Sinai, CorEvitas (formerly Corrona); P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; H. Marzo-Ortega, None.

