Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0974–1003) SLE – Treatment Poster II
0976: Low-dose Belimumab and Antimalarial Agents Prevent Renal Flares in Systemic Lupus Erythematosus: Results from Four Randomised Clinical Trials
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- AG
Alvaro Gomez, MD
Karolinska Institutet
Stockholm, Sweden
Abstract Poster Presenter(s)
Alvaro Gomez1, Sandra Jägerback1, Christopher Sjöwall2 and Ioannis Parodis3, 1Division of Rheumatology, Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden, 2Department of Biomedical and Clinical Sciences, Division of Inflammation and Infection, Linköping University, Linköping, Sweden, 3Karolinska Institutet, Stockholm, Sweden
Background/Purpose: Renal flares contribute substantially to morbidity, renal survival and death in systemic lupus erythematosus (SLE). Identification of pharmacological strategies for the prevention of renal flares is a key unmet need in SLE treatment. In the present study, we tested the hypothesis that the use of belimumab and antimalarial agents (AMA) protects against the development of renal flares.
Methods: We pooled data from the BLISS-52, BLISS-76, BLISS-SC and BLISS-Northeast Asia (NEA) randomised clinical trials of belimumab (N=3225). Serologically active SLE patients with active disease were recruited and followed for 52 weeks in BLISS-52, BLISS-SC and BLISS-NEA and for 76 weeks in BLISS-76; patients with active severe lupus nephritis (LN) were excluded. Patients were allocated to receive intravenous (IV) belimumab 1 mg/kg (N=559), IV belimumab 10 mg/kg (N=1033), SC belimumab 200 mg (N=556) or placebo (N=1077) in addition to standard therapy. The outcome of the present post-hoc analysis was development of renal flares, defined as a reproducible (i) increase in proteinuria to >1 g/day if the baseline value was < 0.2 g/d; >2 g/day if the baseline value was 0.2–1.0 g/d; or >2 times the baseline value if this was >1g/d, (ii) increase in serum creatinine ≥20% or 0.3 mg/dL, accompanied by proteinuria, haematuria or red blood cell (RBC) casts, or (iii) new haematuria of glomerular origin, accompanied by proteinuria or RBC casts. The hazard of renal flare was assessed with Cox proportional hazards regression models. Analyses were adjusted for age, sex, ethnicity, previous renal involvement, baseline proteinuria and glomerular filtration rate, and use of glucocorticoids and immunosuppressants.
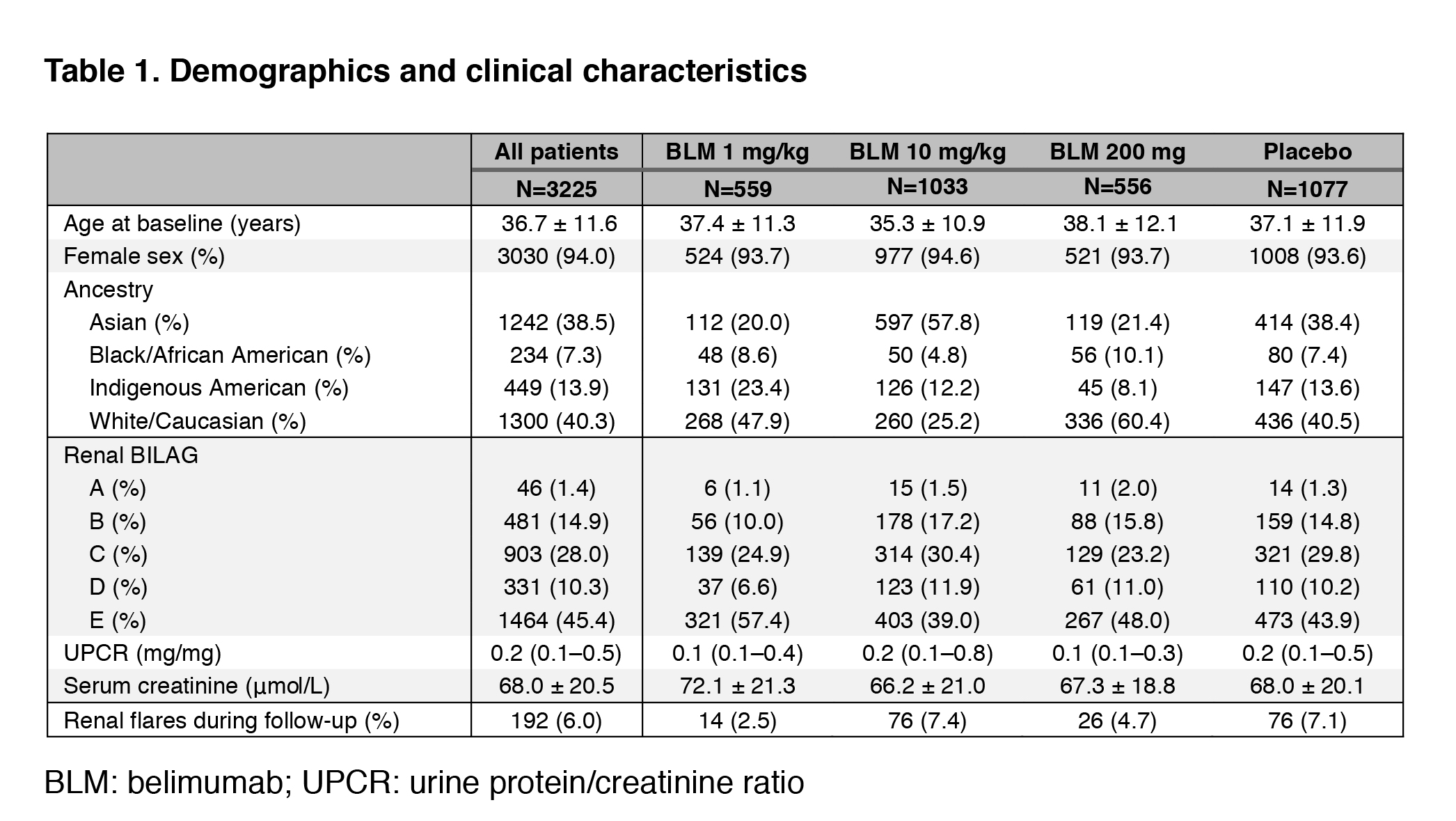
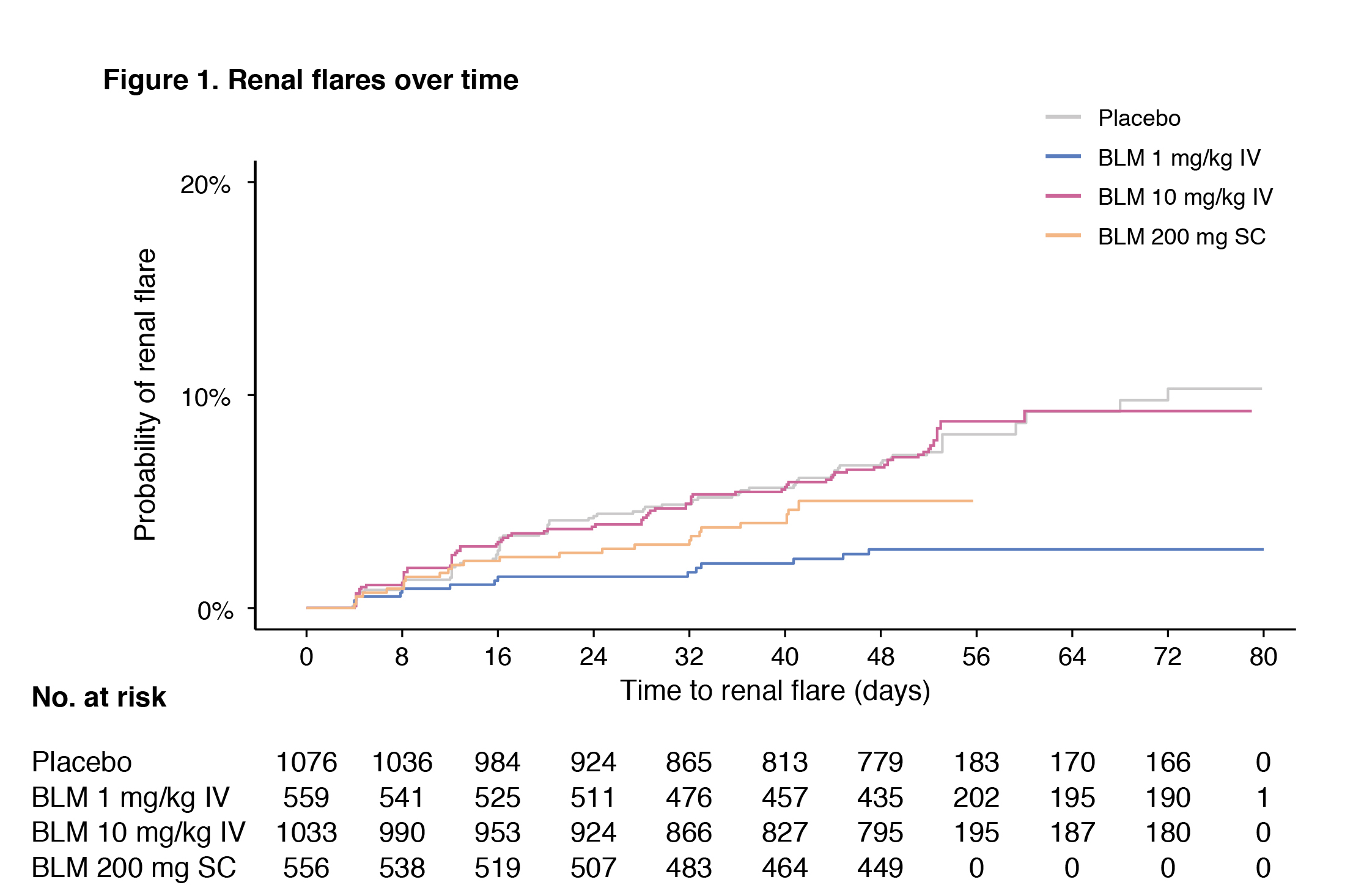
Results: Demographic and clinical characteristics are shown in Table 1. The proportion of patients presenting a renal BILAG A–D at baseline was 54.6%. In the pooled population, 192 patients developed a renal flare after a median of 141 days. In multivariable Cox regression analysis, use of AMA was associated with a lower risk of renal flares (hazard ratio [HR]: 0.66; 95% confidence interval [CI]: 0.49–0.88; p=0.005). Compared with placebo, the risk of renal flares was lower among patients receiving IV belimumab 1 mg/kg (HR: 0.45; 95% CI: 0.25–0.81; p=0.007), but not IV belimumab 10 mg/kg (HR: 0.79; 95% CI: 0.57–1.09; p=0.148) or SC belimumab 200 mg (HR: 0.93; 95% CI: 0.58–1.47; p=0.749). In subgroup analyses including patients with renal BILAG A–D at baseline, these negative associations with renal flares held true for the use of AMA (HR: 0.67; 95% CI: 0.49–0.92; p=0.013) and IV belimumab 1 mg/kg (HR: 0.45; 95% CI: 0.24–0.87; p=0.017).
Conclusion: Low-dose belimumab and AMA were independently associated with a lower risk of renal flare in patients with active SLE from a clinical trial setting. Add-on high-dose belimumab has been approved for the treatment of active LN; the inability to show a protective effect of high-dose belimumab against renal flares in the present study implies that proteinuria levels may have to be considered for dose-adjustment of belimumab in clinical practice, and that investigation of the effect of intermediate doses of belimumab bears merit.
Disclosures: A. Gomez, None; S. Jägerback, AstraZeneca; C. Sjöwall, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche.
Background/Purpose: Renal flares contribute substantially to morbidity, renal survival and death in systemic lupus erythematosus (SLE). Identification of pharmacological strategies for the prevention of renal flares is a key unmet need in SLE treatment. In the present study, we tested the hypothesis that the use of belimumab and antimalarial agents (AMA) protects against the development of renal flares.
Methods: We pooled data from the BLISS-52, BLISS-76, BLISS-SC and BLISS-Northeast Asia (NEA) randomised clinical trials of belimumab (N=3225). Serologically active SLE patients with active disease were recruited and followed for 52 weeks in BLISS-52, BLISS-SC and BLISS-NEA and for 76 weeks in BLISS-76; patients with active severe lupus nephritis (LN) were excluded. Patients were allocated to receive intravenous (IV) belimumab 1 mg/kg (N=559), IV belimumab 10 mg/kg (N=1033), SC belimumab 200 mg (N=556) or placebo (N=1077) in addition to standard therapy. The outcome of the present post-hoc analysis was development of renal flares, defined as a reproducible (i) increase in proteinuria to >1 g/day if the baseline value was < 0.2 g/d; >2 g/day if the baseline value was 0.2–1.0 g/d; or >2 times the baseline value if this was >1g/d, (ii) increase in serum creatinine ≥20% or 0.3 mg/dL, accompanied by proteinuria, haematuria or red blood cell (RBC) casts, or (iii) new haematuria of glomerular origin, accompanied by proteinuria or RBC casts. The hazard of renal flare was assessed with Cox proportional hazards regression models. Analyses were adjusted for age, sex, ethnicity, previous renal involvement, baseline proteinuria and glomerular filtration rate, and use of glucocorticoids and immunosuppressants.
Results: Demographic and clinical characteristics are shown in Table 1. The proportion of patients presenting a renal BILAG A–D at baseline was 54.6%. In the pooled population, 192 patients developed a renal flare after a median of 141 days. In multivariable Cox regression analysis, use of AMA was associated with a lower risk of renal flares (hazard ratio [HR]: 0.66; 95% confidence interval [CI]: 0.49–0.88; p=0.005). Compared with placebo, the risk of renal flares was lower among patients receiving IV belimumab 1 mg/kg (HR: 0.45; 95% CI: 0.25–0.81; p=0.007), but not IV belimumab 10 mg/kg (HR: 0.79; 95% CI: 0.57–1.09; p=0.148) or SC belimumab 200 mg (HR: 0.93; 95% CI: 0.58–1.47; p=0.749). In subgroup analyses including patients with renal BILAG A–D at baseline, these negative associations with renal flares held true for the use of AMA (HR: 0.67; 95% CI: 0.49–0.92; p=0.013) and IV belimumab 1 mg/kg (HR: 0.45; 95% CI: 0.24–0.87; p=0.017).
Conclusion: Low-dose belimumab and AMA were independently associated with a lower risk of renal flare in patients with active SLE from a clinical trial setting. Add-on high-dose belimumab has been approved for the treatment of active LN; the inability to show a protective effect of high-dose belimumab against renal flares in the present study implies that proteinuria levels may have to be considered for dose-adjustment of belimumab in clinical practice, and that investigation of the effect of intermediate doses of belimumab bears merit.


Disclosures: A. Gomez, None; S. Jägerback, AstraZeneca; C. Sjöwall, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche.

