Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0913–0938) RA – Treatment Poster II
0915: Methotrexate Therapy Results in Disparate Methotrexate Polyglutamate Profiles in Peripheral Blood Mononuclear Cells Compared with Erythrocytes
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Renske Hebing, MSc
Reade
Amsterdam, Netherlands
Abstract Poster Presenter(s)
Renske Hebing1, Marry Lin2, Sohaila Mahmoud3, Ittai B. Muller2, Sandra Heil4, Pieter Griffioen4, Eduard A. Struys2, Willem Lems5, Bart van den Bemt6, Michael Nurmohamed7, Gerrit Jansen8 and Robert De Jonge9, 1Amsterdam Rheumatology and immunology Center, Amsterdam UMC – location Reade, Amsterdam, Netherlands, 2Department of Clinical Chemistry, Amsterdam University Medical Centers – location VUMC, Amsterdam, Netherlands, 3Amsterdam Rheumatology and immunology Center, location Reade, Amsterdam, Netherlands, 4Department of Clinical Chemistry, Erasmus MC, Rotterdam, Netherlands, 5Amsterdam University Medical Centers, Amsterdam, Netherlands, 6Department of Pharmacy, Sint Maartenskliniek, Ubbergen, Netherlands, 7Amsterdam University Medical Center, Kortenhoef, Netherlands, 8Department of Rheumatology and Clinical Immunology, Amsterdam University Medical Centers – location VUMC, Amsterdam, Netherlands, 9Department of Clinical Chemistry, Amsterdam University Medical Centers, Amsterdam, Netherlands
Background/Purpose: Analyses of methotrexate polyglutamates (MTX-PGs) in red blood cells (RBCs) has been employed as tool for personalized therapy approach for RA patients during MTX therapy. However, rather than RBCs, peripheral blood mononuclear cells (PBMCs) represent the fraction of immune-effector cells primarily targeted by MTX. Thus far, no prospective study has been performed measuring MTX-PG levels in PBMCs vs RBCs in the early phase of MTX treatment. Here, we investigated the pharmacokinetics of MTX-PG accumulation in RBCs and PBMCs in newly diagnosed RA patients in the early phase (1, 2, 3 and 6 months) of oral and subcutaneous MTX treatment and its relationship with disease activity.
Methods: In a clinical prospective cohort study (Methotrexate Monitoring (MeMo) study), newly diagnosed RA patients (n=46, mean DAS28: 3.5) were randomized for oral or subcutaneous MTX as described before [1]. At 1, 2, 3 and 6 months after start of therapy, blood was collected and RBCs and PBMCs isolated. MTX-PG1-6 concentrations were analyzed using a validated UPLC-MS/MS method with stable isotopes of MTX-PG1-6 as internal standards [2].
Results: PBMCs and RBCs revealed a disparate profile in both absolute MTX-PG accumulation levels and distribution profile (Fig 1&2). Total intracellular MTX-PG1-6 accumulation in PBMCs was significantly (p< 0.001) 10-20-fold higher than RBCs at all time points, regardless of the administration route (Fig 1). MTX-PG distribution in PBMCs was for 85-90% composed of short chain MTX-PG1-3 (PG1>PG2>PG3) and a remainder fraction of MTX-PG4-6. Remarkably, the distribution profile in PBMCs remained constant over a period of 6 months (Fig 2). This profile is distinct from the profile in RBCs. Irrespective of the administration route (without corrections for covariates), RBCs contained mainly MTX-PG1 and lower levels of MTX-PG2-5 at 1 month after therapy start. (Results of a multivariate model of MTX-PGs in RBCs in this cohort have been published before [1].) After 3 months of therapy, MTX-PG3 was the main PG-moiety in RBCs. This profile is largely retained after 6 months of MTX therapy. Conceivably, the disparate MTX-PG accumulation and distribution profiles in PBMCs versus RBCs of RA patients may be associated with the shorter life span of PBMCs and their higher enzymatic activity of folylpolyglutamate synthetase, which is responsible for the conversion of MTX to MTX-PGs [3]. Based on EULAR response criteria, MTX-responding patients had higher MTX-PG3 (data not shown) and total RBC MTX-PG levels compared with non-responders (univariate linear mixed effects models, β 0.006, 95% CI 0.00-0.12, p=0.040, Fig 3). This was not seen in PBMCs (data not shown).
Conclusion: Newly diagnosed RA patients starting MTX therapy accumulated significantly 10-20 fold higher MTX-PG concentrations in PBMCs than in RBCs over a period of 6 months, independent of the administration route.MTX-PG distribution profiles differed between PBMC (highest MTX-PG1) and RBCs (highest MTX-PG3).
References:[1] R Hebing et al, Arthritis Rheum (2021):73(Suppl 10); [2] E den Boer et al, Anal Bioanal Chem (2013);405:1673-81; [3] I Muller et al, Ther Drug Monit (2019);41:598-606.
.jpg) Figure 1. Total MTX-PG concentrations in PBMCs (left) and RBCs (right) of RA patients during the first 6 months of oral (orange) or subcutaneous (green) MTX administration. At 6 months, 18 patients using oral and 18 patients using subcutaneous MTX were still continuing MTX treatment. Note the different scaling of the Y-axis.
Figure 1. Total MTX-PG concentrations in PBMCs (left) and RBCs (right) of RA patients during the first 6 months of oral (orange) or subcutaneous (green) MTX administration. At 6 months, 18 patients using oral and 18 patients using subcutaneous MTX were still continuing MTX treatment. Note the different scaling of the Y-axis.
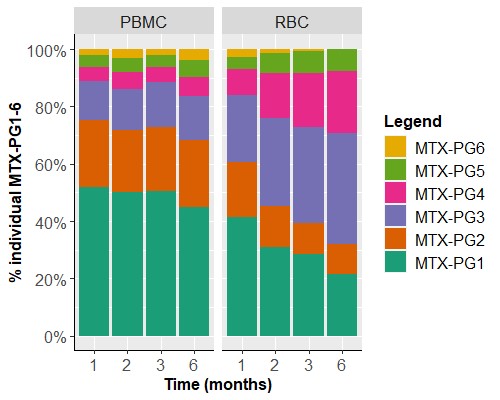 Figure 2. MTX-PG subspecies distributions in PBMCs (left) and RBCs (right) during the first 6 months of combined oral and subcutaneous MTX treatment with MTX-PG1 being most abundant in PBMCs and accumulating MTX-PG3 in RBCs over time.
Figure 2. MTX-PG subspecies distributions in PBMCs (left) and RBCs (right) during the first 6 months of combined oral and subcutaneous MTX treatment with MTX-PG1 being most abundant in PBMCs and accumulating MTX-PG3 in RBCs over time.
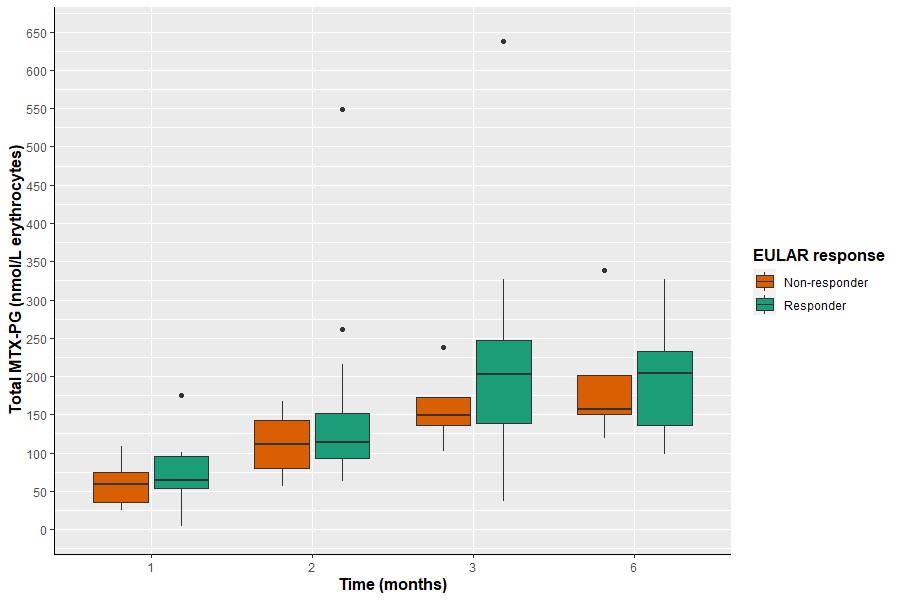 Figure 3. MTX-PG total in RBCs versus response with responders tending to have higher MTX-PG total levels compared with non-responders.
Figure 3. MTX-PG total in RBCs versus response with responders tending to have higher MTX-PG total levels compared with non-responders.
Disclosures: R. Hebing, None; M. Lin, None; S. Mahmoud, None; I. Muller, None; S. Heil, None; P. Griffioen, None; E. Struys, None; W. Lems, None; B. van den Bemt, None; M. Nurmohamed, AbbVie/Abbott; G. Jansen, None; R. De Jonge, None.
Background/Purpose: Analyses of methotrexate polyglutamates (MTX-PGs) in red blood cells (RBCs) has been employed as tool for personalized therapy approach for RA patients during MTX therapy. However, rather than RBCs, peripheral blood mononuclear cells (PBMCs) represent the fraction of immune-effector cells primarily targeted by MTX. Thus far, no prospective study has been performed measuring MTX-PG levels in PBMCs vs RBCs in the early phase of MTX treatment. Here, we investigated the pharmacokinetics of MTX-PG accumulation in RBCs and PBMCs in newly diagnosed RA patients in the early phase (1, 2, 3 and 6 months) of oral and subcutaneous MTX treatment and its relationship with disease activity.
Methods: In a clinical prospective cohort study (Methotrexate Monitoring (MeMo) study), newly diagnosed RA patients (n=46, mean DAS28: 3.5) were randomized for oral or subcutaneous MTX as described before [1]. At 1, 2, 3 and 6 months after start of therapy, blood was collected and RBCs and PBMCs isolated. MTX-PG1-6 concentrations were analyzed using a validated UPLC-MS/MS method with stable isotopes of MTX-PG1-6 as internal standards [2].
Results: PBMCs and RBCs revealed a disparate profile in both absolute MTX-PG accumulation levels and distribution profile (Fig 1&2). Total intracellular MTX-PG1-6 accumulation in PBMCs was significantly (p< 0.001) 10-20-fold higher than RBCs at all time points, regardless of the administration route (Fig 1). MTX-PG distribution in PBMCs was for 85-90% composed of short chain MTX-PG1-3 (PG1>PG2>PG3) and a remainder fraction of MTX-PG4-6. Remarkably, the distribution profile in PBMCs remained constant over a period of 6 months (Fig 2). This profile is distinct from the profile in RBCs. Irrespective of the administration route (without corrections for covariates), RBCs contained mainly MTX-PG1 and lower levels of MTX-PG2-5 at 1 month after therapy start. (Results of a multivariate model of MTX-PGs in RBCs in this cohort have been published before [1].) After 3 months of therapy, MTX-PG3 was the main PG-moiety in RBCs. This profile is largely retained after 6 months of MTX therapy. Conceivably, the disparate MTX-PG accumulation and distribution profiles in PBMCs versus RBCs of RA patients may be associated with the shorter life span of PBMCs and their higher enzymatic activity of folylpolyglutamate synthetase, which is responsible for the conversion of MTX to MTX-PGs [3]. Based on EULAR response criteria, MTX-responding patients had higher MTX-PG3 (data not shown) and total RBC MTX-PG levels compared with non-responders (univariate linear mixed effects models, β 0.006, 95% CI 0.00-0.12, p=0.040, Fig 3). This was not seen in PBMCs (data not shown).
Conclusion: Newly diagnosed RA patients starting MTX therapy accumulated significantly 10-20 fold higher MTX-PG concentrations in PBMCs than in RBCs over a period of 6 months, independent of the administration route.MTX-PG distribution profiles differed between PBMC (highest MTX-PG1) and RBCs (highest MTX-PG3).
References:[1] R Hebing et al, Arthritis Rheum (2021):73(Suppl 10); [2] E den Boer et al, Anal Bioanal Chem (2013);405:1673-81; [3] I Muller et al, Ther Drug Monit (2019);41:598-606.
.jpg) Figure 1. Total MTX-PG concentrations in PBMCs (left) and RBCs (right) of RA patients during the first 6 months of oral (orange) or subcutaneous (green) MTX administration. At 6 months, 18 patients using oral and 18 patients using subcutaneous MTX were still continuing MTX treatment. Note the different scaling of the Y-axis.
Figure 1. Total MTX-PG concentrations in PBMCs (left) and RBCs (right) of RA patients during the first 6 months of oral (orange) or subcutaneous (green) MTX administration. At 6 months, 18 patients using oral and 18 patients using subcutaneous MTX were still continuing MTX treatment. Note the different scaling of the Y-axis.  Figure 2. MTX-PG subspecies distributions in PBMCs (left) and RBCs (right) during the first 6 months of combined oral and subcutaneous MTX treatment with MTX-PG1 being most abundant in PBMCs and accumulating MTX-PG3 in RBCs over time.
Figure 2. MTX-PG subspecies distributions in PBMCs (left) and RBCs (right) during the first 6 months of combined oral and subcutaneous MTX treatment with MTX-PG1 being most abundant in PBMCs and accumulating MTX-PG3 in RBCs over time.  Figure 3. MTX-PG total in RBCs versus response with responders tending to have higher MTX-PG total levels compared with non-responders.
Figure 3. MTX-PG total in RBCs versus response with responders tending to have higher MTX-PG total levels compared with non-responders.Disclosures: R. Hebing, None; M. Lin, None; S. Mahmoud, None; I. Muller, None; S. Heil, None; P. Griffioen, None; E. Struys, None; W. Lems, None; B. van den Bemt, None; M. Nurmohamed, AbbVie/Abbott; G. Jansen, None; R. De Jonge, None.

