Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0629–0670) SLE – Etiology and Pathogenesis Poster
0648: Pathogenic Anti-C1q Antibodies Potentiate Activation of the Classical Complement Pathway in a Subset of Lupus Nephritis Patients
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- EC
Edmund Chang, PhD
Annexon Biosciences
Brisbane, CA, United States
Abstract Poster Presenter(s)
Edmund Chang1, Julian Low2, Ellen Cahir-McFarland2, Henk-Andre Kroon2, Yaisa Andrews-Zwilling2, Ted Yednock2 and Ann Mongan2, 1Annexon Biosciences, Brisbane, CA, 2Annexon Biosciences, South San Francisco, CA
Background/Purpose: In LN, excess immune complexes lead to nephrotic deposition of C1q and C4d, the latter of which has been correlated with circulating C4d and C4 levels. The objective of this analysis was to evaluate the relationship among C4d levels, C1q activation, and pathogenic anti-C1q antibodies (PACA) in order to identify which patients are most likely to have disease mediated by classical complement cascade. Such patients would be potential candidates for anti-C1q therapy, such as ANX009 (NCT04535752).
Methods: A panel of complement factors were measured using ELISA from plasma samples from a cohort of 40 patients with LN in the California Lupus Epidemiology Study and 20 healthy controls. All patients included in the LN cohort either had confirmed LN (class III, IV, or V) on their kidney biopsy or had a clinical diagnosis of LN from their rheumatologist or nephrologist. SLE diagnoses were confirmed by study physicians according to either of the following definitions: (a) meeting ≥4 of the 11 ACR revised classification criteria for SLE as defined in 1982 and updated in 1997 or (b) meeting 3 of the 11 ACR criteria along with having SLE confirmed by a study rheumatologist. Clinical disease activity was measured using the SLEDAI, and proteinuria was evaluated by random spot urine protein-to-creatinine ratio.
Results: We observed evidence of activation in the classical complement pathway in patients with LN. Levels of C4d and the C4d/C4 ratio were highly increased in LN patients with flare, while levels of C1q, C1s, and C4 were decreased. C4d/C4 correlated with levels of PACA isotypes 1 and 3, which are known to activate the classical pathway. Changes in C4 and C4d/C4 were associated with improvements in proteinuria and SLEDAI following treatment for disease flare, indicating their potential value as biomarkers of treatment response. The stability of C4d/C4 as a predictive biomarker for ANX009 and the prevalence of LN patients with complement-mediated disease were further confirmed with an independent cohort.
Conclusion: A subset of patients with LN exhibited high C4d/C4 along with specific markers of classical pathway activation, indicative of classical complement pathway disease mediation. Reduction in this ratio appeared to correlate with treatment response, but C4d/C4 values were generally not normalized, suggesting an insufficient resolution of complement-mediated inflammation by current treatments. Our data support a clinical hypothesis that a subset of patients with LN may benefit from a precision medicine approach targeting this pathway. This hypothesis is being evaluated in a clinical trial testing the subcutaneously administered C1q inhibitor ANX009 in patients with active LN.
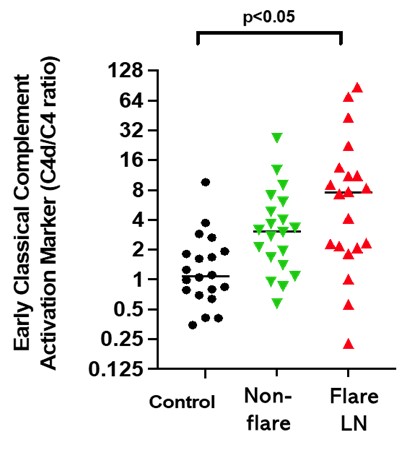 Figure 1. Higher C4d/C4 Ratios Were Found in LN Patients Compared to Normal Controls
Figure 1. Higher C4d/C4 Ratios Were Found in LN Patients Compared to Normal Controls
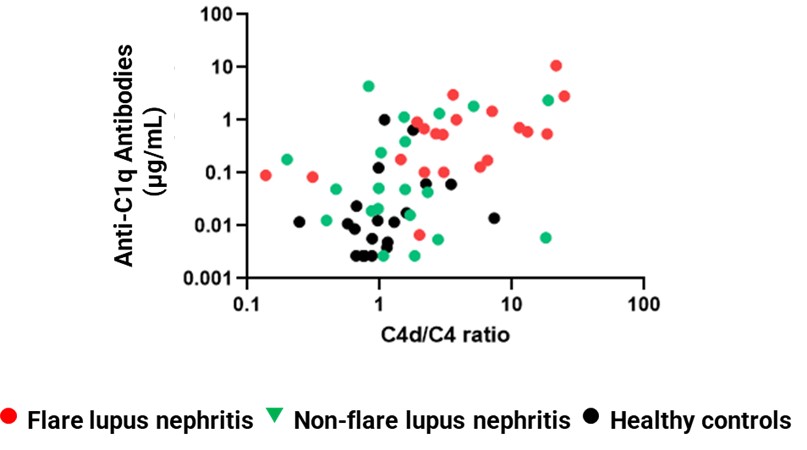 Figure 2. C4d/C4 Correlates With Anti-C1q Antibodies
Figure 2. C4d/C4 Correlates With Anti-C1q Antibodies
Disclosures: E. Chang, Annexon Biosciences, Gilead Sciences; J. Low, Annexon Biosciences, Bristol Myers Squibb; E. Cahir-McFarland, Annexon Biosciences, Biogen; H. Kroon, Annexon Biosciences; Y. Andrews-Zwilling, Annexon Biosciences; T. Yednock, Annexon Biosciences, Cortexyme; A. Mongan, Annexon Biosciences.
Background/Purpose: In LN, excess immune complexes lead to nephrotic deposition of C1q and C4d, the latter of which has been correlated with circulating C4d and C4 levels. The objective of this analysis was to evaluate the relationship among C4d levels, C1q activation, and pathogenic anti-C1q antibodies (PACA) in order to identify which patients are most likely to have disease mediated by classical complement cascade. Such patients would be potential candidates for anti-C1q therapy, such as ANX009 (NCT04535752).
Methods: A panel of complement factors were measured using ELISA from plasma samples from a cohort of 40 patients with LN in the California Lupus Epidemiology Study and 20 healthy controls. All patients included in the LN cohort either had confirmed LN (class III, IV, or V) on their kidney biopsy or had a clinical diagnosis of LN from their rheumatologist or nephrologist. SLE diagnoses were confirmed by study physicians according to either of the following definitions: (a) meeting ≥4 of the 11 ACR revised classification criteria for SLE as defined in 1982 and updated in 1997 or (b) meeting 3 of the 11 ACR criteria along with having SLE confirmed by a study rheumatologist. Clinical disease activity was measured using the SLEDAI, and proteinuria was evaluated by random spot urine protein-to-creatinine ratio.
Results: We observed evidence of activation in the classical complement pathway in patients with LN. Levels of C4d and the C4d/C4 ratio were highly increased in LN patients with flare, while levels of C1q, C1s, and C4 were decreased. C4d/C4 correlated with levels of PACA isotypes 1 and 3, which are known to activate the classical pathway. Changes in C4 and C4d/C4 were associated with improvements in proteinuria and SLEDAI following treatment for disease flare, indicating their potential value as biomarkers of treatment response. The stability of C4d/C4 as a predictive biomarker for ANX009 and the prevalence of LN patients with complement-mediated disease were further confirmed with an independent cohort.
Conclusion: A subset of patients with LN exhibited high C4d/C4 along with specific markers of classical pathway activation, indicative of classical complement pathway disease mediation. Reduction in this ratio appeared to correlate with treatment response, but C4d/C4 values were generally not normalized, suggesting an insufficient resolution of complement-mediated inflammation by current treatments. Our data support a clinical hypothesis that a subset of patients with LN may benefit from a precision medicine approach targeting this pathway. This hypothesis is being evaluated in a clinical trial testing the subcutaneously administered C1q inhibitor ANX009 in patients with active LN.
 Figure 1. Higher C4d/C4 Ratios Were Found in LN Patients Compared to Normal Controls
Figure 1. Higher C4d/C4 Ratios Were Found in LN Patients Compared to Normal Controls Figure 2. C4d/C4 Correlates With Anti-C1q Antibodies
Figure 2. C4d/C4 Correlates With Anti-C1q Antibodies Disclosures: E. Chang, Annexon Biosciences, Gilead Sciences; J. Low, Annexon Biosciences, Bristol Myers Squibb; E. Cahir-McFarland, Annexon Biosciences, Biogen; H. Kroon, Annexon Biosciences; Y. Andrews-Zwilling, Annexon Biosciences; T. Yednock, Annexon Biosciences, Cortexyme; A. Mongan, Annexon Biosciences.

