Back
Poster Session B
Reproductive health
Session: (0939–0969) Reproductive Issues in Rheumatic Disorders Poster
0939: Pilot Study: A Novel Method for Cervical Health Monitoring in African American Women with Systemic Lupus Erythematosus (SLE) Using a Self- Sampling Brush to Assess Cervical HPV Infection and Cervical Cytology
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- JD
J. Patricia Dhar, MD
Ascension St. John Hospital and Wayne State Univeristy School of Medicine
Bloomfield Hills, MI, United States
Abstract Poster Presenter(s)
J. Patricia Dhar1, Heather Walline2, Lamia Fathallah3, Susanna Szpunar4, Louis Saravolatz5, Gil Mor6 and Thomas Carey7, 1Ascension St. John Hospital and Wayne State University School of Medicine, Bloomfield Hills, MI, 2University of Michigan, Ann Arbor, MI, 3Ascension St.John Hospital, Detroit, MI, 4Ascension St. John Hospital, Grosse Pointe Woods, MI, 5Ascension St John Hospital and Medical Center, Wayne State University School of Medicine (affiliate), Grosse Pointe Woods, MI, 6Wayne State University and C.S. Mott Center for Human Growth and Development., Detroit, MI, 7University of Michigan School of Medicine, Ann Arbor, MI
Background/Purpose: A health disparity exists for AA women with SLE who have increased morbidity & mortality from both cervical cancer & SLE. Current cervical cancer screening guidelines are inadequate for these women who need frequent monitoring. We pilot tested a self-sampling brush to obtain cervical cytology & study HPV genetics to develop a less expensive & more convenient way to make cervical cancer screening more accessible for these high risk women
Methods: 30 AA women with SLE recruited from the Rheumatology clinics consented to participate in the study (IRB approved by Ascension St. John Hospital IRB). Inclusion criteria: AA women with SLE, ages 18-50 years, able to consent and obtain vaginal self sampling. Exclusion criteria: current pregnancy, & not meeting inclusion criteria. Clinical information was obtained by records review & interviewing the patient, & surveys administered to assess cervical health information, knowledge of HPV, and satisfaction with using the self sampling brush. Vaginal self sampling by the participant was performed after instruction & the brush immersed in an RNA-stable ThinPrep vial. Hologic ThinPrep automated processor was used for slide preparation & cytology read by a board-certified cytopathologist. HPV DNA &RNA were extracted, & samples were frozen at -80 0C for further analysis.
Results: All patients met established criteria for SLE: mean age =36.9 yrs., 40% had lupus nephritis, & all treated with chronic immunosuppressives [Table 1]. Two thirds had a history of abnormal pap smear ranging from atypical squamous sells of undermined significance (ASCUS) to high grade squamous intra-epithelial lesion (HSIL)/cervical intra-epithelial lesion 3 (CIN 3); 7 had a cervical biopsy & 6 underwent a surgical procedure for abnormal cytology or genital warts. HPV test results were available in 43% of which 8 were positive.Most participants had sexually transmitted diseases other than HPV, were not vaccinated for HPV, had multiple lifetime sex partners, did not smoke, & did not use condoms. The average time to last pap smear was 3.2 yrs.[Table 2] Most had knowledge that HPV can cause cervical cancer, but were unaware of HPV causing oral & genital cancers & warts. Most felt the device was easy to use, comfortable, & preferred it for over traditional pap smear. [Table 3].Cytology samples obtained from the brush showed preservation of morphology, but quality indicators were generally absent with abnormal findings in 4/30 : 3=low grade squamous intra-epithelial lesion (LSIL) and 1=ASCUS. Participants were notified of abnormal results & advised to see a gynecologist.
Conclusion: Our high risk cohort had histories of adverse cervical health, yet lacked knowledge about the spectrum of HPV related genital & oral disease & were not following health prevention strategies to reduce their risk with HPV vaccination and condoms. Cervical cytology can be performed using a brush self sampling method of collection. This study highlights the need for cervical health education, increased monitoring & intervention in these high risk women.
.jpg) Table 1
Table 1
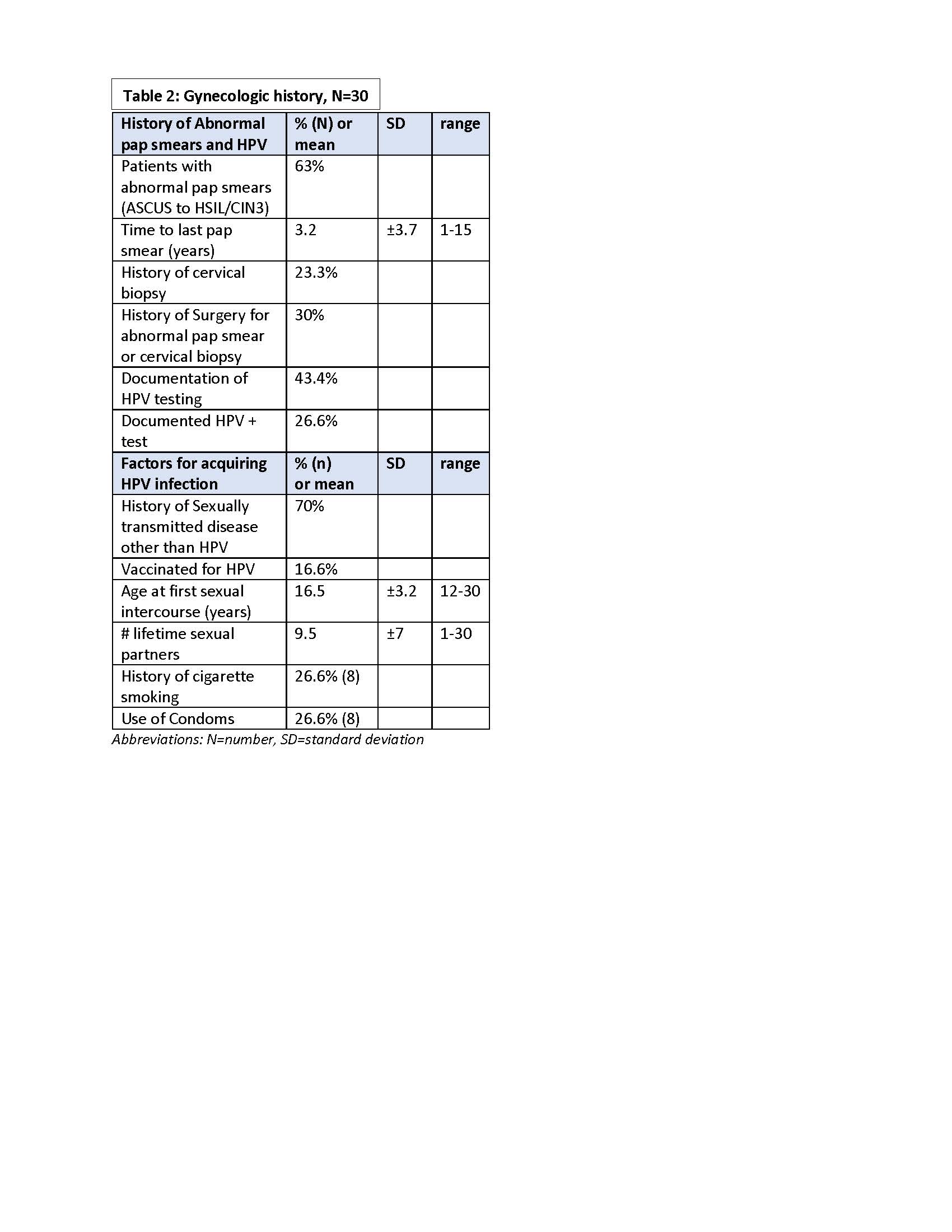 Table 2
Table 2
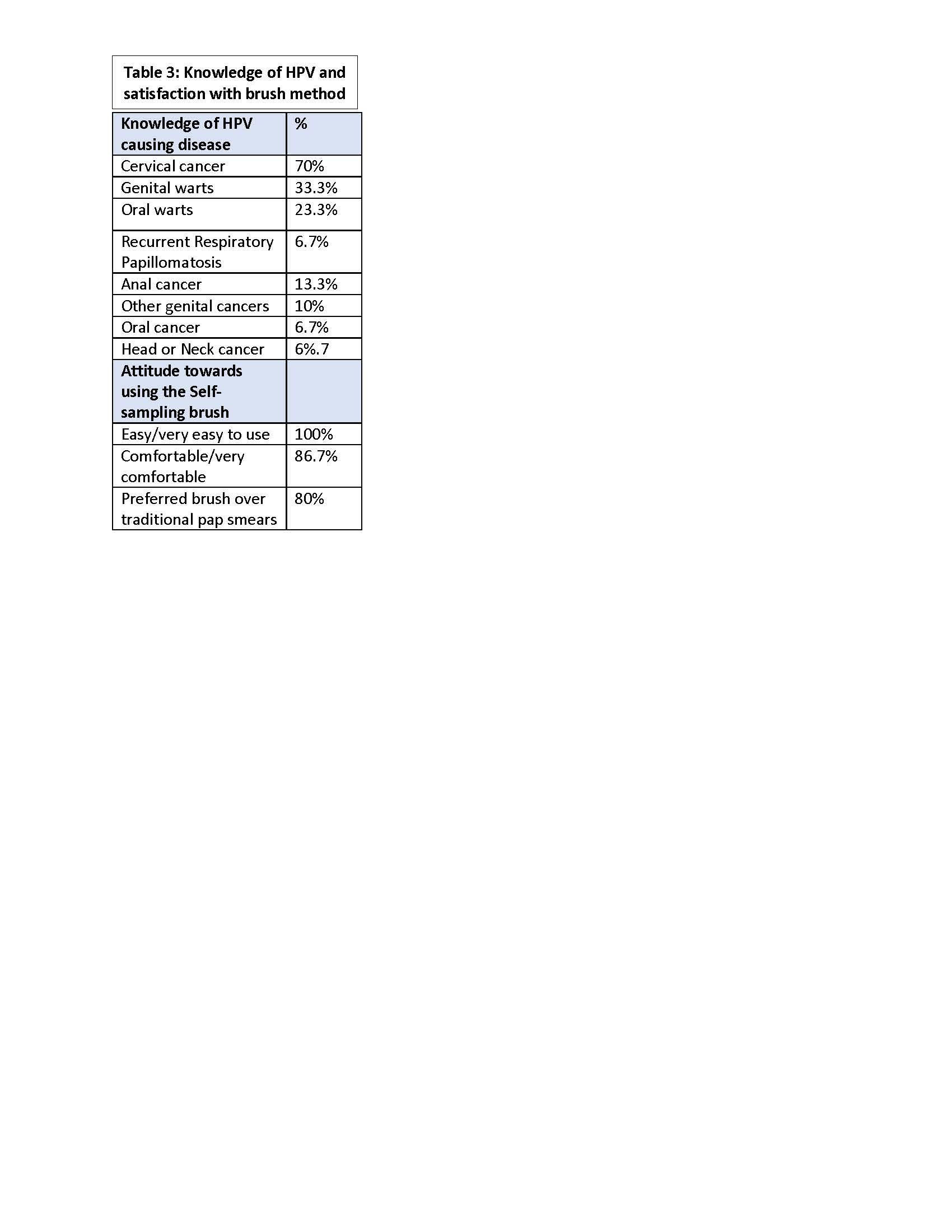 Table 3
Table 3
Disclosures: J. Dhar, None; H. Walline, None; L. Fathallah, None; S. Szpunar, None; L. Saravolatz, None; G. Mor, None; T. Carey, None.
Background/Purpose: A health disparity exists for AA women with SLE who have increased morbidity & mortality from both cervical cancer & SLE. Current cervical cancer screening guidelines are inadequate for these women who need frequent monitoring. We pilot tested a self-sampling brush to obtain cervical cytology & study HPV genetics to develop a less expensive & more convenient way to make cervical cancer screening more accessible for these high risk women
Methods: 30 AA women with SLE recruited from the Rheumatology clinics consented to participate in the study (IRB approved by Ascension St. John Hospital IRB). Inclusion criteria: AA women with SLE, ages 18-50 years, able to consent and obtain vaginal self sampling. Exclusion criteria: current pregnancy, & not meeting inclusion criteria. Clinical information was obtained by records review & interviewing the patient, & surveys administered to assess cervical health information, knowledge of HPV, and satisfaction with using the self sampling brush. Vaginal self sampling by the participant was performed after instruction & the brush immersed in an RNA-stable ThinPrep vial. Hologic ThinPrep automated processor was used for slide preparation & cytology read by a board-certified cytopathologist. HPV DNA &RNA were extracted, & samples were frozen at -80 0C for further analysis.
Results: All patients met established criteria for SLE: mean age =36.9 yrs., 40% had lupus nephritis, & all treated with chronic immunosuppressives [Table 1]. Two thirds had a history of abnormal pap smear ranging from atypical squamous sells of undermined significance (ASCUS) to high grade squamous intra-epithelial lesion (HSIL)/cervical intra-epithelial lesion 3 (CIN 3); 7 had a cervical biopsy & 6 underwent a surgical procedure for abnormal cytology or genital warts. HPV test results were available in 43% of which 8 were positive.Most participants had sexually transmitted diseases other than HPV, were not vaccinated for HPV, had multiple lifetime sex partners, did not smoke, & did not use condoms. The average time to last pap smear was 3.2 yrs.[Table 2] Most had knowledge that HPV can cause cervical cancer, but were unaware of HPV causing oral & genital cancers & warts. Most felt the device was easy to use, comfortable, & preferred it for over traditional pap smear. [Table 3].Cytology samples obtained from the brush showed preservation of morphology, but quality indicators were generally absent with abnormal findings in 4/30 : 3=low grade squamous intra-epithelial lesion (LSIL) and 1=ASCUS. Participants were notified of abnormal results & advised to see a gynecologist.
Conclusion: Our high risk cohort had histories of adverse cervical health, yet lacked knowledge about the spectrum of HPV related genital & oral disease & were not following health prevention strategies to reduce their risk with HPV vaccination and condoms. Cervical cytology can be performed using a brush self sampling method of collection. This study highlights the need for cervical health education, increased monitoring & intervention in these high risk women.
.jpg) Table 1
Table 1 Table 2
Table 2 Table 3
Table 3Disclosures: J. Dhar, None; H. Walline, None; L. Fathallah, None; S. Szpunar, None; L. Saravolatz, None; G. Mor, None; T. Carey, None.

