Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0792: Post-mRNA Vaccine Flares in Autoimmune Inflammatory Rheumatic Diseases: Results from the COronavirus National Vaccine Registry for ImmuNe Diseases SINGapore (CONVIN-SING)
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- ML
Manjari Lahiri, MD, MBBS, MRCP
National University Hospital
Singapore, Singapore
Abstract Poster Presenter(s)
Margaret Ma1, Amelia Santosa1, Kok Ooi Kong2, Chuanhui Xu2, Gim Gee Teng1, Johnston GX Tang2, Anselm Mak3, Frank Tay1, Victoria WW Ng4, Joshua ZE Koh4, Warren Fong5, Li-Ching Chew6, Andrea Low7, annie law8, Yih Jia Poh8, Siaw Ing Yeo6, Ying Ying Leung9, Wei-Rui Goh6, Nur Emilia Roslan10, Tyng Yu Chuah10, Stanley Angkodjojo11, Thaschawee Arkachaisri12, Kai Liang Teh13, Kee Fong Phang14, Melonie Sriranganathan15, teck Choon Tan16, Peter Cheung1 and Manjari Lahiri1, 1National University Hospital, Singapore, Singapore, 2Tan Tock Seng Hospital, Singapore, Singapore, 3Division of Rheumatology, Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 4National University of Singapore, Singapore, Singapore, 5Singapore Health Services Pte Ltd (SHS), Singapore, Singapore, 6Singapore General Hospital, Singapore, Singapore, 7Department of Rheumatology and Immunology, Singapore General Hospital, Singapore, Singapore, 8SingHealth, Singapore, Singapore, 9Rheumatology Department, Singapore General Hospital, Singapore, Singapore, 10Sengkang General Hospital, Singapore, Singapore, 11Sengkang General Hospital / Singhealth, Singapore, Singapore, 12KK Women's and Children's Hospital, SingHealth, Singapore, Singapore, 13KK Women's and Children's Hospital, Singapore, Singapore, 14Alexandra Hospital, Singapore, Singapore, 15Changi General Hospital, Singapore, Singapore, 16Khoo Teck Puat Hospital, Singapore, Singapore
Background/Purpose: Published data suggest no increased rate of flare of autoimmune inflammatory rheumatic diseases (AIIRD) after COVID-19 mRNA vaccination; however, the studies are limited by small sample size, short follow up or at risk of selection bias (voluntary physician reports or patient surveys). Therefore, our aim is to study flares of AIIRD within three months of the first dose of an anti-SARS-COV2 mRNA vaccine.
Methods: A retrospective cohort study of consecutive AIIRD patients ≥ 12 years old, across 8 public hospitals in Singapore who received at least one dose of an mRNA (Pfizer/BioNTech or Moderna) vaccine. Data were censored at the first post-vaccine clinic visit when the patient had flared or if ≥ three months had elapsed since the first dose of the vaccine, whichever came first. Predictors of flare were determined by Cox proportional hazards analysis and time to flare was examined using a Nelson Aalen cumulative hazard estimate (Figure 1).
Results: 4627 patients (73.3% Chinese, 71.1% female) of median (IQR) age 61 (48, 70) years were included in the analysis (Table). 4058 (87.7%) had the Pfizer/BioNTech vaccine and 523 (11.3%) had Moderna, with a median (IQR) interval of 21 (21, 28) days between the two doses. The most common AIIRD diagnoses were Rheumatoid arthritis (1966, 42.4%), Psoriatic arthritis (486, 10.5%) and Systemic lupus erythematosus (SLE) (641, 13.8%). 2188 (47.3%) patients were in remission, 1897 (41%) in low disease activity, 480 (10.4%) in moderate disease activity and 62 (1.3%) in high disease activity. 431 (9.3%) were treated with biologics/ targeted disease modifying agents. Treatment was interrupted for vaccination in only 63 (1.4 %) patients. 45 (1%) patients had previous COVID-19 infection. 854 (18%) flares were recorded during 20,287 patient-months (4,5/100 patient-months, median (IQR) follow up duration 4.3 (3.4, 5.5) months), of which 542 (11.7%) patients flared in the 3-month period of interest. Median (IQR) time to flare was 40 (18, 56) days (Figure 1). 134 (24.7%) flares were mild and self-limiting, 333 (61.4%) were mild-moderate and 75 (13.8%) were severe. 393 (72.5%) of those who flared required escalation of treatment and 30 (5.5%) required hospital admission. 545 (11.8%) had improved disease activity after the vaccine.
On multivariate Cox regression analysis, patients in the oldest age tertile [median (IQR) 72 (69, 77) years] were less likely to flare [HR 0.76 (95% CI 0.64, 0.90), p = 0.001] Patients with inflammatory arthritis (compared with connective tissue disease, vasculitis and others) and patients with baseline active disease were more likely to flare [HR 1.88 (95% CI 1.56, 2.25), p < 0.001 and 1.54 (95% CI 1.28, 1.85), p < 0.001 respectively] Gender, ethnicity and vaccine type were not significant predictors of flare. (Figure 2)
Conclusion: There was a moderately high rate of AIIRD flares after mRNA vaccination; however, there was no clustering of flares in the immediate post-vaccine period to suggest causality and severe flares were rare. Older patients were less likely to flare, while those with inflammatory arthritis and active disease at baseline were more likely to flare.
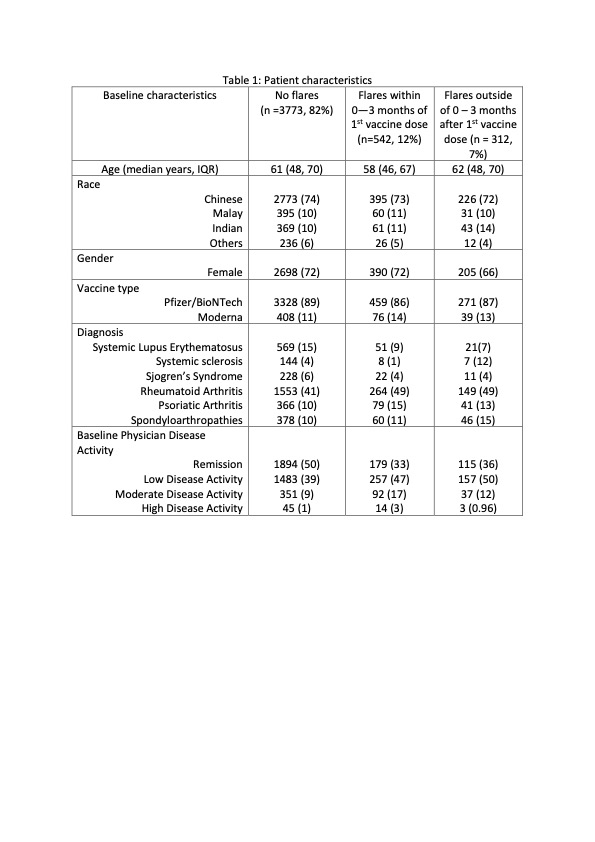 Table 1: Patient characteristics
Table 1: Patient characteristics
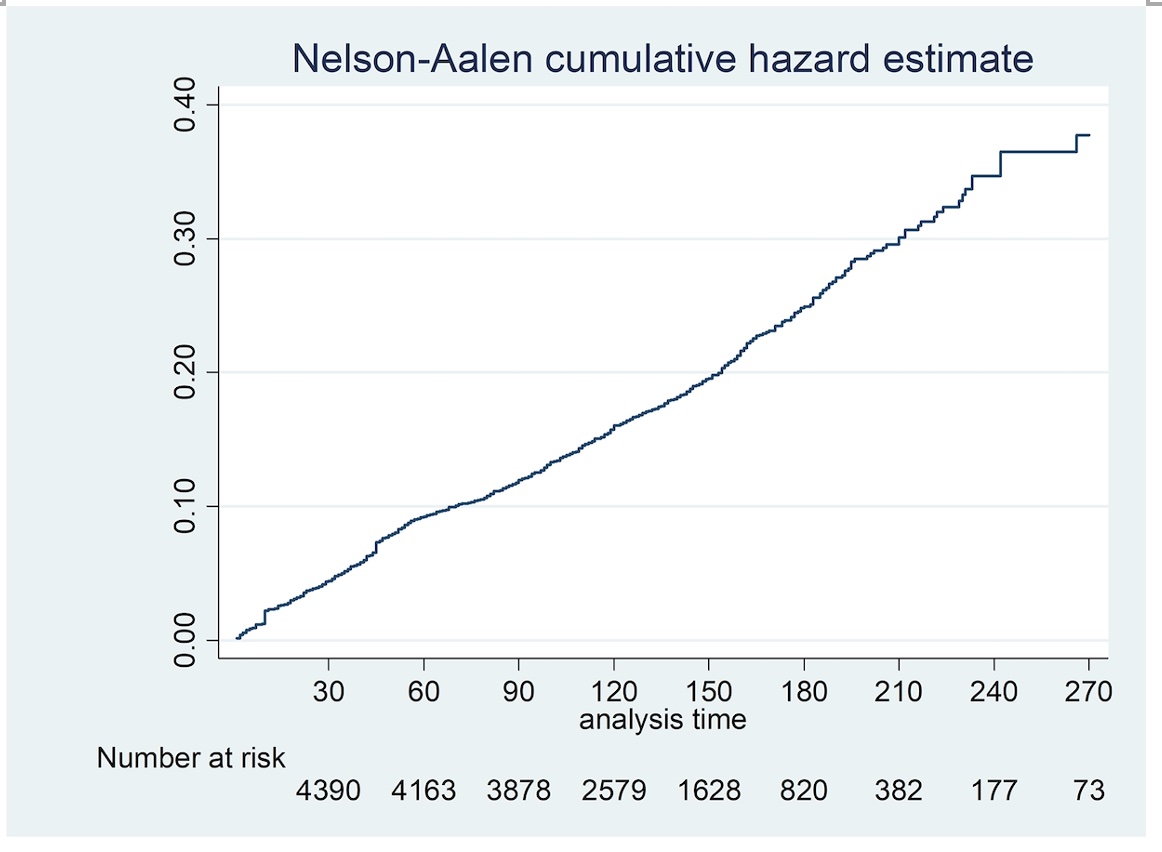 Figure 1: Time to flare was examined using a Nelson Aalen cumulative hazard estimate
Figure 1: Time to flare was examined using a Nelson Aalen cumulative hazard estimate
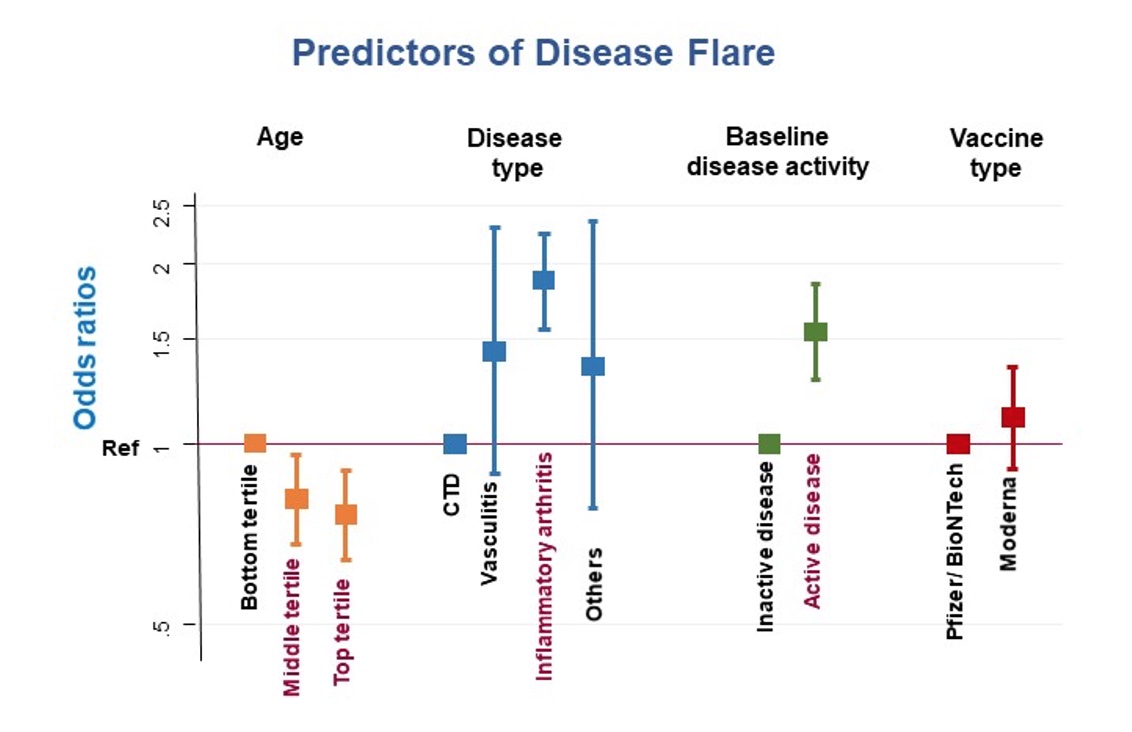 Figure 2: Predictors of disease flare using Cox proportional hazards analysis
Figure 2: Predictors of disease flare using Cox proportional hazards analysis
Disclosures: M. Ma, Janssen, AbbVie/Abbott, Pfizer; A. Santosa, None; K. Kong, None; C. Xu, None; G. Teng, None; J. Tang, None; A. Mak, None; F. Tay, None; V. Ng, None; J. Koh, None; W. Fong, Novartis, Janssen, GlaxoSmithKlein(GSK); L. Chew, Pfizer, Pfizer, AbbVie/Abbott, Pfizer, AbbVie/Abbott, AbbVie/Abbott; A. Low, None; a. law, None; Y. Poh, None; S. Yeo, AbbVie/Abbott, Pfizer, Janssen; Y. Leung, AbbVie/Abbott, Janssen, Pfizer, DKSH; W. Goh, None; N. Roslan, None; T. Chuah, None; S. Angkodjojo, None; T. Arkachaisri, None; K. Teh, None; K. Phang, None; M. Sriranganathan, None; t. Tan, None; P. Cheung, Janssen, Boehringer-Ingelheim, Novartis, AbbVie/Abbott; M. Lahiri, AbbVie/Abbott, Janssen, DKSH.
Background/Purpose: Published data suggest no increased rate of flare of autoimmune inflammatory rheumatic diseases (AIIRD) after COVID-19 mRNA vaccination; however, the studies are limited by small sample size, short follow up or at risk of selection bias (voluntary physician reports or patient surveys). Therefore, our aim is to study flares of AIIRD within three months of the first dose of an anti-SARS-COV2 mRNA vaccine.
Methods: A retrospective cohort study of consecutive AIIRD patients ≥ 12 years old, across 8 public hospitals in Singapore who received at least one dose of an mRNA (Pfizer/BioNTech or Moderna) vaccine. Data were censored at the first post-vaccine clinic visit when the patient had flared or if ≥ three months had elapsed since the first dose of the vaccine, whichever came first. Predictors of flare were determined by Cox proportional hazards analysis and time to flare was examined using a Nelson Aalen cumulative hazard estimate (Figure 1).
Results: 4627 patients (73.3% Chinese, 71.1% female) of median (IQR) age 61 (48, 70) years were included in the analysis (Table). 4058 (87.7%) had the Pfizer/BioNTech vaccine and 523 (11.3%) had Moderna, with a median (IQR) interval of 21 (21, 28) days between the two doses. The most common AIIRD diagnoses were Rheumatoid arthritis (1966, 42.4%), Psoriatic arthritis (486, 10.5%) and Systemic lupus erythematosus (SLE) (641, 13.8%). 2188 (47.3%) patients were in remission, 1897 (41%) in low disease activity, 480 (10.4%) in moderate disease activity and 62 (1.3%) in high disease activity. 431 (9.3%) were treated with biologics/ targeted disease modifying agents. Treatment was interrupted for vaccination in only 63 (1.4 %) patients. 45 (1%) patients had previous COVID-19 infection. 854 (18%) flares were recorded during 20,287 patient-months (4,5/100 patient-months, median (IQR) follow up duration 4.3 (3.4, 5.5) months), of which 542 (11.7%) patients flared in the 3-month period of interest. Median (IQR) time to flare was 40 (18, 56) days (Figure 1). 134 (24.7%) flares were mild and self-limiting, 333 (61.4%) were mild-moderate and 75 (13.8%) were severe. 393 (72.5%) of those who flared required escalation of treatment and 30 (5.5%) required hospital admission. 545 (11.8%) had improved disease activity after the vaccine.
On multivariate Cox regression analysis, patients in the oldest age tertile [median (IQR) 72 (69, 77) years] were less likely to flare [HR 0.76 (95% CI 0.64, 0.90), p = 0.001] Patients with inflammatory arthritis (compared with connective tissue disease, vasculitis and others) and patients with baseline active disease were more likely to flare [HR 1.88 (95% CI 1.56, 2.25), p < 0.001 and 1.54 (95% CI 1.28, 1.85), p < 0.001 respectively] Gender, ethnicity and vaccine type were not significant predictors of flare. (Figure 2)
Conclusion: There was a moderately high rate of AIIRD flares after mRNA vaccination; however, there was no clustering of flares in the immediate post-vaccine period to suggest causality and severe flares were rare. Older patients were less likely to flare, while those with inflammatory arthritis and active disease at baseline were more likely to flare.
 Table 1: Patient characteristics
Table 1: Patient characteristics Figure 1: Time to flare was examined using a Nelson Aalen cumulative hazard estimate
Figure 1: Time to flare was examined using a Nelson Aalen cumulative hazard estimate  Figure 2: Predictors of disease flare using Cox proportional hazards analysis
Figure 2: Predictors of disease flare using Cox proportional hazards analysisDisclosures: M. Ma, Janssen, AbbVie/Abbott, Pfizer; A. Santosa, None; K. Kong, None; C. Xu, None; G. Teng, None; J. Tang, None; A. Mak, None; F. Tay, None; V. Ng, None; J. Koh, None; W. Fong, Novartis, Janssen, GlaxoSmithKlein(GSK); L. Chew, Pfizer, Pfizer, AbbVie/Abbott, Pfizer, AbbVie/Abbott, AbbVie/Abbott; A. Low, None; a. law, None; Y. Poh, None; S. Yeo, AbbVie/Abbott, Pfizer, Janssen; Y. Leung, AbbVie/Abbott, Janssen, Pfizer, DKSH; W. Goh, None; N. Roslan, None; T. Chuah, None; S. Angkodjojo, None; T. Arkachaisri, None; K. Teh, None; K. Phang, None; M. Sriranganathan, None; t. Tan, None; P. Cheung, Janssen, Boehringer-Ingelheim, Novartis, AbbVie/Abbott; M. Lahiri, AbbVie/Abbott, Janssen, DKSH.

