Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0974–1003) SLE – Treatment Poster II
0991: Prior Use of Autoimmune Disease Treatments Among Patients with Systemic Lupus Erythematosus, Rheumatoid Arthritis, or Myositis Hospitalized with COVID-19
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
.jpg)
Cassandra Calabrese, DO
Cleveland Clinic Foundation
Cleveland Heights, OH, United States
Abstract Poster Presenter(s)
Cassandra Calabrese1, Gelareh Atefi2, Kristin Evans3, Meghan Moynihan3, Liisa Palmer3 and Sandra Sze-jung Wu4, 1Cleveland Clinic Foundation, Cleveland Heights, OH, 2AstraZeneca, Wilmington, DE, 3Merative, Cambridge, MA, 4AstraZeneca, Hockessin, DE
Background/Purpose: To compare use of autoimmune disease treatments between patients with and without severe COVID-19 (COVID) in cohorts of patients with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), or myositis (MYO).
Methods: Healthcare claims in the IBM® MarketScan® Commercial Database were used to identify adults (18+) with a confirmed diagnosis of SLE, RA, or MYO between 1/1/2013 and 4/1/2020. Observation began on 4/1/2020 and ended with the earliest of enrollment end, death, or 12/31/2021. Severe COVID was identified as an inpatient (IP) claim with a COVID ICD-10 diagnosis code (U071 or J1282). Use of antimalarials, biologics, and immunosuppressants was assessed in the 6 months before a patient's earliest COVID diagnosis, and corticosteroid use was assessed in the prior 30 days. For patients without severe COVID, medication use was assessed in the 6 months (or 30 days) before a proxy date, assigned based on a matched distribution of earliest diagnosis dates among patients with severe COVID. Medication use was not assessed for 17% of SLE patients without severe COVID whose assigned proxy date was outside of their observation period. Demographics were assessed on 4/1/2020. SLE disease severity and diagnoses in the Elixhauser Comorbidity Index were assessed among patients enrolled during the 12 months before 4/1/2020. Unadjusted comparisons between patients with and without severe COVID were made with chi-square or Fisher's exact tests.
Results: 18,665 patients with SLE, 52,406 with RA, and 1,833 with MYO met inclusion criteria. Severe COVID occurred among 1.5% of SLE, 1.4% of RA, 2.2% of MYO patients. Patients with severe COVID were older in all three cohorts, and SLE and RA patients with severe COVID were more likely to be male, compared to patients without severe COVID (Table 1). Comorbidities were significantly more common among patients with severe COVID in all three cohorts. Patients with severe COVID in each disease cohort were significantly more likely to have previously used any medication (79-83%), compared to patients without severe COVID (62-75%) (Figure 1). Prior use of any biologic, immunosuppressant, or corticosteroid was also significantly more common among patients with severe COVID in each cohort, and use of antimalarials was more common among RA patients with severe COVID. SLE, RA, and MYO patients with severe COVID were more likely to have used rituximab (7-20%) or mycophenolate (3-40%), compared to patients without severe COVID (2-6% and 1-15%, respectively) (Figure 2). Belimumab was more common among SLE patients with vs. without severe COVID (12% vs. 8%), and leflunomide and tacrolimus were more common among SLE and RA patients with vs. without severe COVID (SLE: 4% vs. 2% and 8% vs. 3%; RA: 12% vs. 6% and 1% vs. 0.3%). No use of anifrolumab was found in the 6-months before severe COVID events.
Conclusion: Some autoimmune disease treatments especially corticosteroids, are more prevalent among SLE, RA, and MYO patients with severe COVID. These treatments may be indicative of autoimmune disease severity, or independently associated with the occurrence of severe COVID. The potential impact on patients' COVID risk should be carefully considered when selecting or adjusting autoimmune treatment regimens.
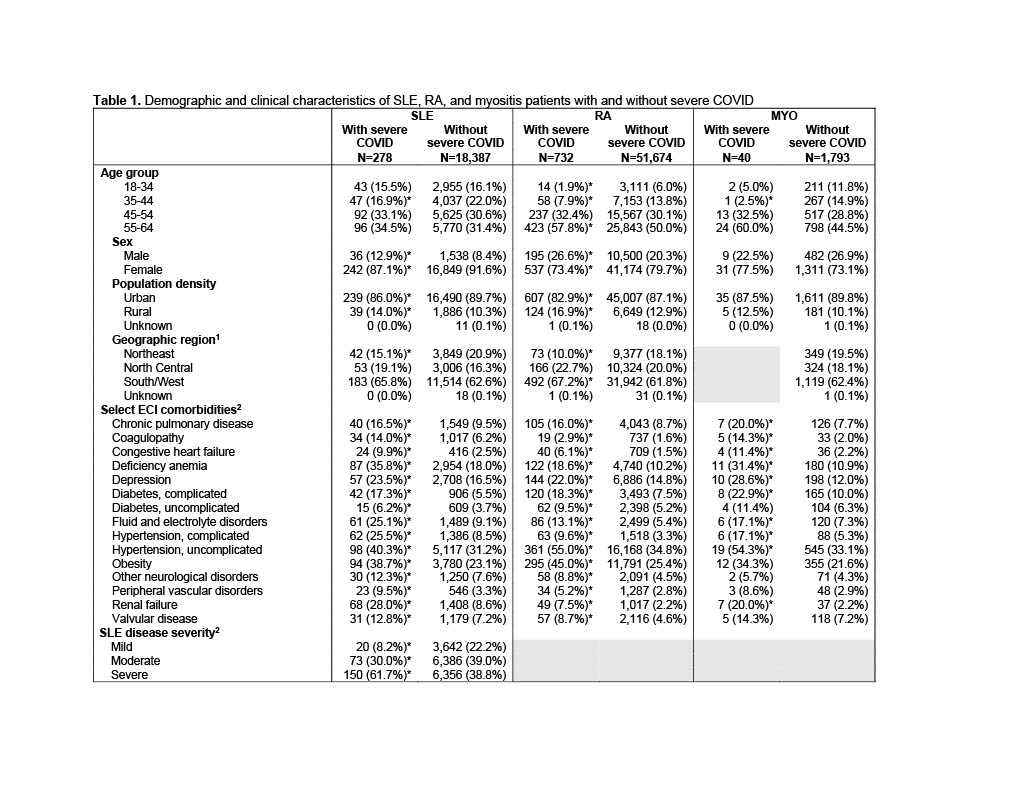 1 Geographic region not reported for myositis patients with severe COVID due to low sample size; 2 Comorbidities and SLE disease severity assessed among patients enrolled during the 12 months before 4/1/2020 (89% of SLE, 90% of RA, 92% of MYO); * p < 0.05 vs. without severe COVID; ECI: Elixhauser Comorbidity index; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
1 Geographic region not reported for myositis patients with severe COVID due to low sample size; 2 Comorbidities and SLE disease severity assessed among patients enrolled during the 12 months before 4/1/2020 (89% of SLE, 90% of RA, 92% of MYO); * p < 0.05 vs. without severe COVID; ECI: Elixhauser Comorbidity index; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
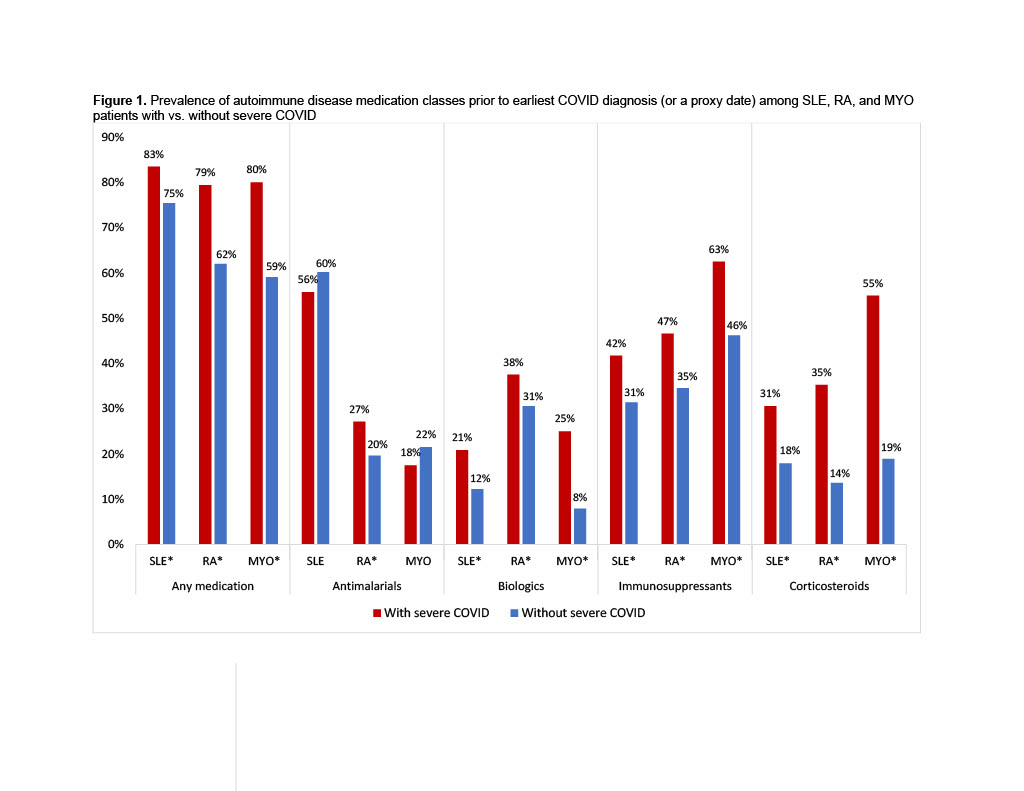 * p < 0.05 vs. without severe COVID; severe COVID defined as an inpatient admission with a COVID diagnosis; use of antimalarials, biologics, and immunosuppressants was assessed in the 6 months before the earliest COVID diagnosis, or before a proxy date for patients without severe COVID; use of corticosteroids assessed in the prior 30 days; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
* p < 0.05 vs. without severe COVID; severe COVID defined as an inpatient admission with a COVID diagnosis; use of antimalarials, biologics, and immunosuppressants was assessed in the 6 months before the earliest COVID diagnosis, or before a proxy date for patients without severe COVID; use of corticosteroids assessed in the prior 30 days; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
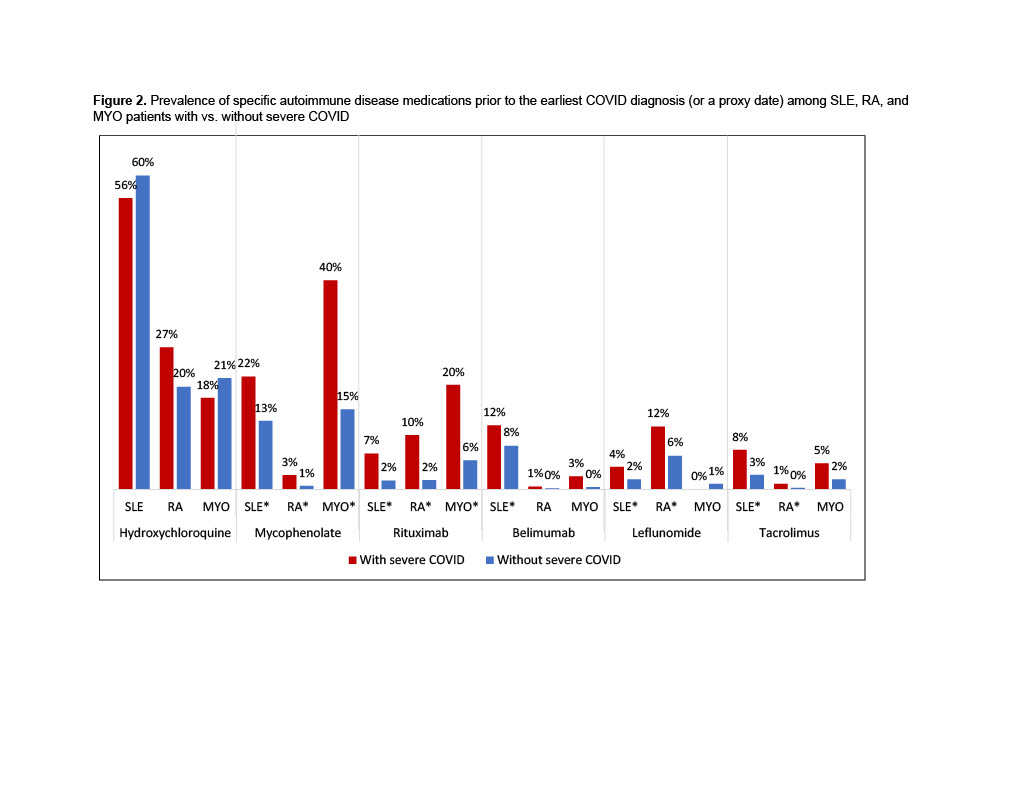 * p < 0.05 vs. without severe COVID; severe COVID defined as an inpatient admission with a COVID diagnosis; use of each medication was assessed in the 6 months before the earliest COVID diagnosis, or before a proxy date for patients without severe COVID; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
* p < 0.05 vs. without severe COVID; severe COVID defined as an inpatient admission with a COVID diagnosis; use of each medication was assessed in the 6 months before the earliest COVID diagnosis, or before a proxy date for patients without severe COVID; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
Disclosures: C. Calabrese, Sanofi, Astrazenica; G. Atefi, AstraZeneca; K. Evans, None; M. Moynihan, None; L. Palmer, None; S. Wu, AstraZeneca.
Background/Purpose: To compare use of autoimmune disease treatments between patients with and without severe COVID-19 (COVID) in cohorts of patients with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), or myositis (MYO).
Methods: Healthcare claims in the IBM® MarketScan® Commercial Database were used to identify adults (18+) with a confirmed diagnosis of SLE, RA, or MYO between 1/1/2013 and 4/1/2020. Observation began on 4/1/2020 and ended with the earliest of enrollment end, death, or 12/31/2021. Severe COVID was identified as an inpatient (IP) claim with a COVID ICD-10 diagnosis code (U071 or J1282). Use of antimalarials, biologics, and immunosuppressants was assessed in the 6 months before a patient's earliest COVID diagnosis, and corticosteroid use was assessed in the prior 30 days. For patients without severe COVID, medication use was assessed in the 6 months (or 30 days) before a proxy date, assigned based on a matched distribution of earliest diagnosis dates among patients with severe COVID. Medication use was not assessed for 17% of SLE patients without severe COVID whose assigned proxy date was outside of their observation period. Demographics were assessed on 4/1/2020. SLE disease severity and diagnoses in the Elixhauser Comorbidity Index were assessed among patients enrolled during the 12 months before 4/1/2020. Unadjusted comparisons between patients with and without severe COVID were made with chi-square or Fisher's exact tests.
Results: 18,665 patients with SLE, 52,406 with RA, and 1,833 with MYO met inclusion criteria. Severe COVID occurred among 1.5% of SLE, 1.4% of RA, 2.2% of MYO patients. Patients with severe COVID were older in all three cohorts, and SLE and RA patients with severe COVID were more likely to be male, compared to patients without severe COVID (Table 1). Comorbidities were significantly more common among patients with severe COVID in all three cohorts. Patients with severe COVID in each disease cohort were significantly more likely to have previously used any medication (79-83%), compared to patients without severe COVID (62-75%) (Figure 1). Prior use of any biologic, immunosuppressant, or corticosteroid was also significantly more common among patients with severe COVID in each cohort, and use of antimalarials was more common among RA patients with severe COVID. SLE, RA, and MYO patients with severe COVID were more likely to have used rituximab (7-20%) or mycophenolate (3-40%), compared to patients without severe COVID (2-6% and 1-15%, respectively) (Figure 2). Belimumab was more common among SLE patients with vs. without severe COVID (12% vs. 8%), and leflunomide and tacrolimus were more common among SLE and RA patients with vs. without severe COVID (SLE: 4% vs. 2% and 8% vs. 3%; RA: 12% vs. 6% and 1% vs. 0.3%). No use of anifrolumab was found in the 6-months before severe COVID events.
Conclusion: Some autoimmune disease treatments especially corticosteroids, are more prevalent among SLE, RA, and MYO patients with severe COVID. These treatments may be indicative of autoimmune disease severity, or independently associated with the occurrence of severe COVID. The potential impact on patients' COVID risk should be carefully considered when selecting or adjusting autoimmune treatment regimens.
 1 Geographic region not reported for myositis patients with severe COVID due to low sample size; 2 Comorbidities and SLE disease severity assessed among patients enrolled during the 12 months before 4/1/2020 (89% of SLE, 90% of RA, 92% of MYO); * p < 0.05 vs. without severe COVID; ECI: Elixhauser Comorbidity index; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
1 Geographic region not reported for myositis patients with severe COVID due to low sample size; 2 Comorbidities and SLE disease severity assessed among patients enrolled during the 12 months before 4/1/2020 (89% of SLE, 90% of RA, 92% of MYO); * p < 0.05 vs. without severe COVID; ECI: Elixhauser Comorbidity index; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus * p < 0.05 vs. without severe COVID; severe COVID defined as an inpatient admission with a COVID diagnosis; use of antimalarials, biologics, and immunosuppressants was assessed in the 6 months before the earliest COVID diagnosis, or before a proxy date for patients without severe COVID; use of corticosteroids assessed in the prior 30 days; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
* p < 0.05 vs. without severe COVID; severe COVID defined as an inpatient admission with a COVID diagnosis; use of antimalarials, biologics, and immunosuppressants was assessed in the 6 months before the earliest COVID diagnosis, or before a proxy date for patients without severe COVID; use of corticosteroids assessed in the prior 30 days; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus * p < 0.05 vs. without severe COVID; severe COVID defined as an inpatient admission with a COVID diagnosis; use of each medication was assessed in the 6 months before the earliest COVID diagnosis, or before a proxy date for patients without severe COVID; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus
* p < 0.05 vs. without severe COVID; severe COVID defined as an inpatient admission with a COVID diagnosis; use of each medication was assessed in the 6 months before the earliest COVID diagnosis, or before a proxy date for patients without severe COVID; MYO: myositis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosusDisclosures: C. Calabrese, Sanofi, Astrazenica; G. Atefi, AstraZeneca; K. Evans, None; M. Moynihan, None; L. Palmer, None; S. Wu, AstraZeneca.

