Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0787: Relationship Between Humoral and Cellular Immune Responses in Persons with Rheumatoid Arthritis Following a Third Dose of COVID-19 Vaccine
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
.png)
Sara Tedeschi, MD, MPH
Brigham and Women's Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Sara Tedeschi1, Daniel Solomon1, Yuezhou Chen2, Jack Ellrodt1, Mary Grace Whelan1, Jacklyn Stratton1, Keigo Hayashi3, Noah Whiteman2, Lin Chen1, Ifeoluwakiisi Adejoorin1, Kathryne Marks1, Emma Gomez-Rivas1, Deepak Rao1, Anna Jonsson1 and Duane Wesemann2, 1Brigham and Women's Hospital, Boston, MA, 2Harvard Medical School, Boston, MA, 3Brigham and Women's Hospital, Okayama, Japan
Background/Purpose: DMARDs that treat RA may reduce immune responses to COVID-19 vaccination. We compared measures of humoral and cell-mediated immunity in RA patients before and after a 3rd dose of COVID-19 vaccine.
Methods: RA patients that had previously received 2 doses of mRNA vaccine enrolled in a single-center prospective observational study, July-Dec 2021, before receiving a 3rd dose of mRNA vaccine. Subjects self-reported holding or continuing DMARDs; rituximab users were excluded from analysis. RA disease activity (RADAI-5) was self-reported weekly pre- and post-3rd dose. Blood samples were collected pre- and week 4 post-3rd dose. Humoral response was measured with in-house ELISA assays for anti-Spike (anti-S) and anti-receptor binding domain (anti-RBD) IgG in equivalent units to monoclonal antibody positive control; results were log-transformed prior to statistical analyses. We calculated delta and fold-change of anti-S and anti-RBD post- vs. pre-3rd dose. "Healthy response" to the 3rd dose was defined as ELISA within 1 standard deviation of the mean among 6 healthy controls 4-8 weeks after a 2nd dose of mRNA vaccine. Cell-mediated response was assessed in a subset of seropositive RA subjects by incubating PBMCs with SARS-CoV-2 peptide pool (Miltenyi Biotec). Adjusted frequencies of stimulated CD4 T cells expressing < ![if !msEquation] >< ![if !vml] > < ![endif] >< ![endif] >2 activation markers (IFNg, TNF, IL-2, 4-1BB, CD40L, CD69) were compared pre- vs. post-3rd dose. Spearman's correlations assessed the relationship between ELISA levels and frequencies of activated CD4 T cells. Subgroup analyses compared subjects (a) holding vs. continuing DMARDs and (b) by DMARD group.
< ![endif] >< ![endif] >2 activation markers (IFNg, TNF, IL-2, 4-1BB, CD40L, CD69) were compared pre- vs. post-3rd dose. Spearman's correlations assessed the relationship between ELISA levels and frequencies of activated CD4 T cells. Subgroup analyses compared subjects (a) holding vs. continuing DMARDs and (b) by DMARD group.
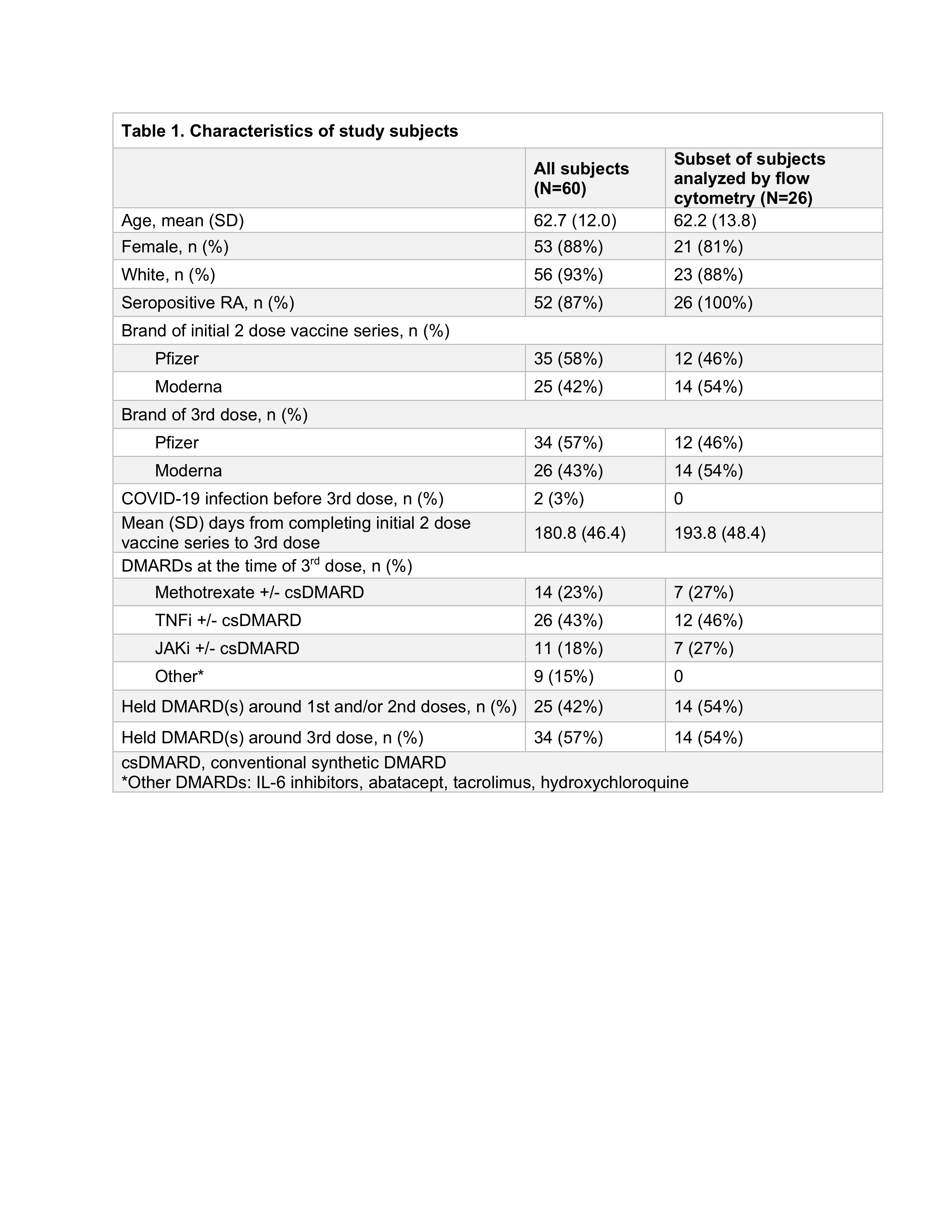
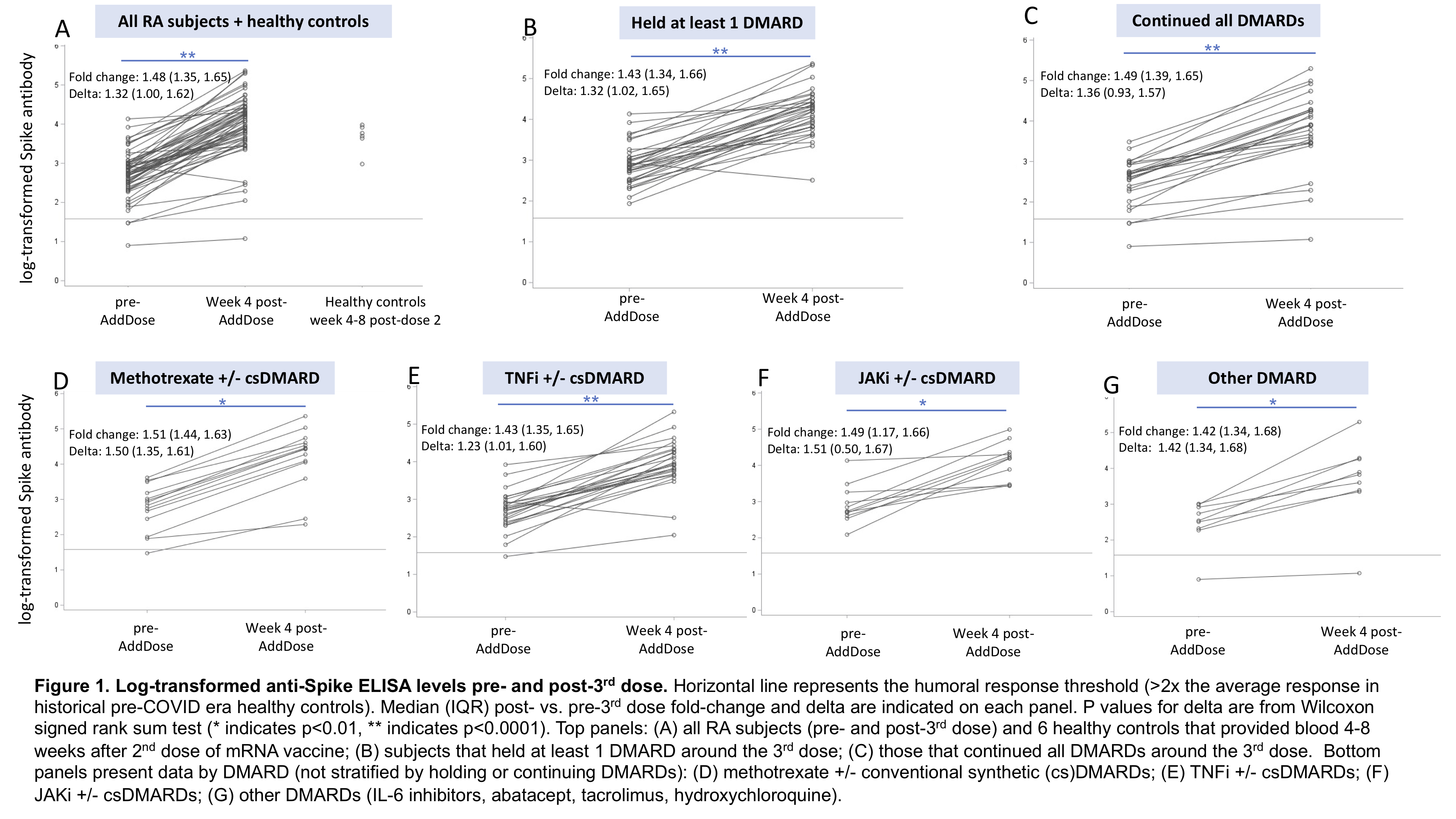
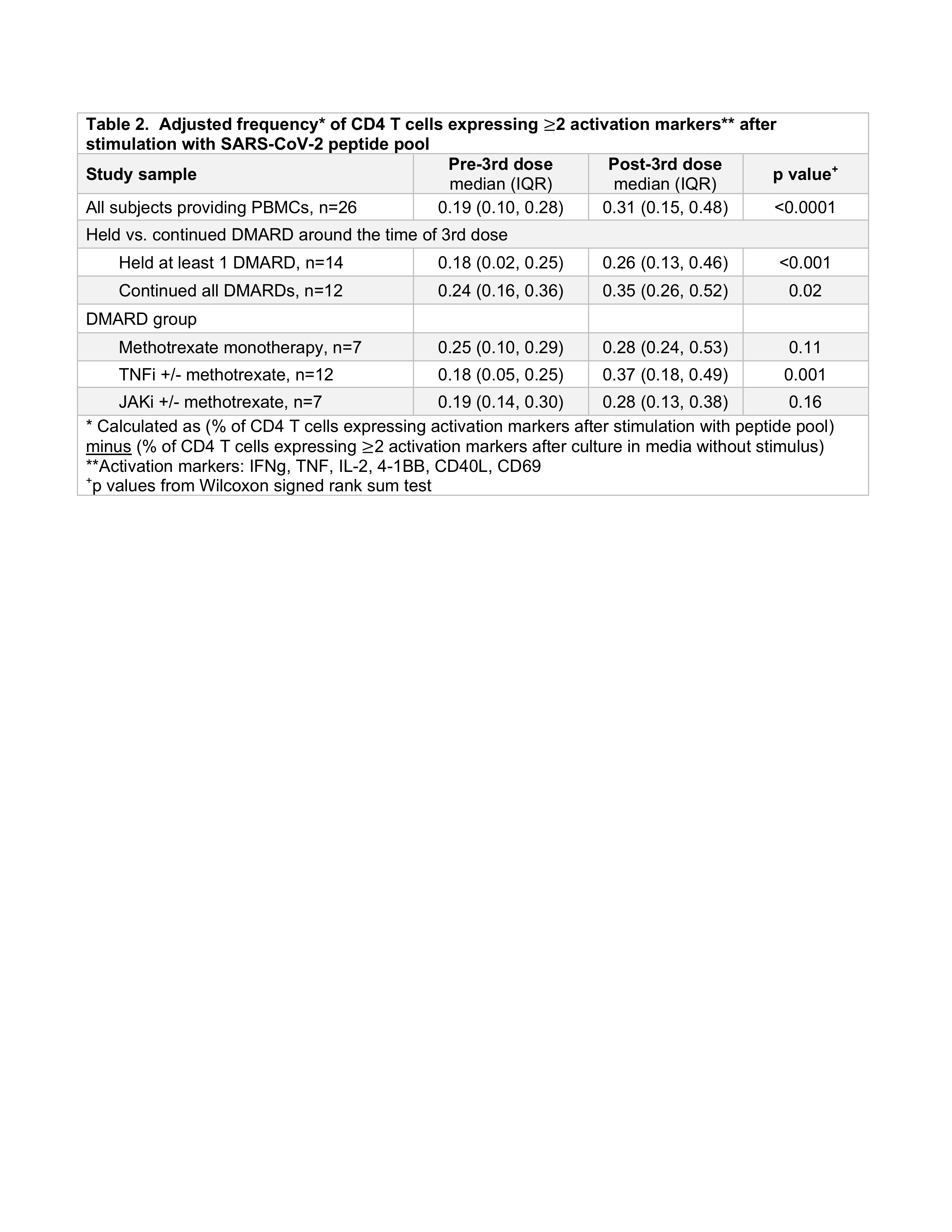
Results: Among 60 subjects, mean age was 63 and 88% were female. TNFi (43%) and methotrexate (23%) were the most common DMARDs (Table 1). 57% held at least 1 DMARD around the 3rd dose; baseline RA disease activity did not significantly differ between those holding vs. continuing DMARDs (p=0.58). At week 4 post-3rd dose, a "healthy response" was observed in 38% for anti-S and 45% for anti-RBD. Anti-S ELISA levels increased 1.4-fold among those holding DMARDs and 1.5-fold among those continuing DMARDs (p=0.56); anti-RBD increased 1.6-fold in those holding DMARDs and 1.7-fold in those continuing DMARDs (p=0.76) (Figure 1). Fold-change in anti-S and anti-RBD ELISA were similar when comparing methotrexate to other DMARD groups (p >0.05 for each comparison). Among 26 subjects that provided PBMCs, frequencies of activated CD4 T cells were significantly greater post- vs. pre-3rd dose in both those that held DMARDs (p< 0.01) and those that continued DMARDs (p=0.02) (Table 2). Neither fold-change nor delta ELISA level was significantly correlated with the change in frequency of activated CD4 T cells (rho -0.26 to -0.06, p >0.05).
Conclusion: After a 3rd dose of mRNA COVID vaccine, ELISA levels significantly increased in RA subjects using DMARDs though fewer than half achieved a humoral response on par with healthy controls after a 2nd dose. Change in ELISA levels did not correlate with CD4 T cell responses. Additional studies are needed to determine clinical relevance of quantitative differences in humoral and cell-mediated responses in immunosuppressed patients.
Disclosures: S. Tedeschi, Moderna, NGM Biopharmaceuticals; D. Solomon, AbbVie/Abbott, Amgen, moderna, CorEvitas; Y. Chen, None; J. Ellrodt, None; M. Whelan, None; J. Stratton, None; K. Hayashi, None; N. Whiteman, None; L. Chen, None; I. Adejoorin, None; K. Marks, None; E. Gomez-Rivas, None; D. Rao, Janssen, Merck, Bristol-Myers Squibb, Scipher Medicine, HiFiBio, Inc., AstraZeneca, Pfizer; A. Jonsson, Amgen; D. Wesemann, None.
Background/Purpose: DMARDs that treat RA may reduce immune responses to COVID-19 vaccination. We compared measures of humoral and cell-mediated immunity in RA patients before and after a 3rd dose of COVID-19 vaccine.
Methods: RA patients that had previously received 2 doses of mRNA vaccine enrolled in a single-center prospective observational study, July-Dec 2021, before receiving a 3rd dose of mRNA vaccine. Subjects self-reported holding or continuing DMARDs; rituximab users were excluded from analysis. RA disease activity (RADAI-5) was self-reported weekly pre- and post-3rd dose. Blood samples were collected pre- and week 4 post-3rd dose. Humoral response was measured with in-house ELISA assays for anti-Spike (anti-S) and anti-receptor binding domain (anti-RBD) IgG in equivalent units to monoclonal antibody positive control; results were log-transformed prior to statistical analyses. We calculated delta and fold-change of anti-S and anti-RBD post- vs. pre-3rd dose. "Healthy response" to the 3rd dose was defined as ELISA within 1 standard deviation of the mean among 6 healthy controls 4-8 weeks after a 2nd dose of mRNA vaccine. Cell-mediated response was assessed in a subset of seropositive RA subjects by incubating PBMCs with SARS-CoV-2 peptide pool (Miltenyi Biotec). Adjusted frequencies of stimulated CD4 T cells expressing < ![if !msEquation] >< ![if !vml] >
Results: Among 60 subjects, mean age was 63 and 88% were female. TNFi (43%) and methotrexate (23%) were the most common DMARDs (Table 1). 57% held at least 1 DMARD around the 3rd dose; baseline RA disease activity did not significantly differ between those holding vs. continuing DMARDs (p=0.58). At week 4 post-3rd dose, a "healthy response" was observed in 38% for anti-S and 45% for anti-RBD. Anti-S ELISA levels increased 1.4-fold among those holding DMARDs and 1.5-fold among those continuing DMARDs (p=0.56); anti-RBD increased 1.6-fold in those holding DMARDs and 1.7-fold in those continuing DMARDs (p=0.76) (Figure 1). Fold-change in anti-S and anti-RBD ELISA were similar when comparing methotrexate to other DMARD groups (p >0.05 for each comparison). Among 26 subjects that provided PBMCs, frequencies of activated CD4 T cells were significantly greater post- vs. pre-3rd dose in both those that held DMARDs (p< 0.01) and those that continued DMARDs (p=0.02) (Table 2). Neither fold-change nor delta ELISA level was significantly correlated with the change in frequency of activated CD4 T cells (rho -0.26 to -0.06, p >0.05).
Conclusion: After a 3rd dose of mRNA COVID vaccine, ELISA levels significantly increased in RA subjects using DMARDs though fewer than half achieved a humoral response on par with healthy controls after a 2nd dose. Change in ELISA levels did not correlate with CD4 T cell responses. Additional studies are needed to determine clinical relevance of quantitative differences in humoral and cell-mediated responses in immunosuppressed patients.



Disclosures: S. Tedeschi, Moderna, NGM Biopharmaceuticals; D. Solomon, AbbVie/Abbott, Amgen, moderna, CorEvitas; Y. Chen, None; J. Ellrodt, None; M. Whelan, None; J. Stratton, None; K. Hayashi, None; N. Whiteman, None; L. Chen, None; I. Adejoorin, None; K. Marks, None; E. Gomez-Rivas, None; D. Rao, Janssen, Merck, Bristol-Myers Squibb, Scipher Medicine, HiFiBio, Inc., AstraZeneca, Pfizer; A. Jonsson, Amgen; D. Wesemann, None.

