Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1004–1034) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
1026: Responsiveness and Minimal Clinically Important Difference in Patient-Reported Outcome Measures Among Patients with Psoriatic Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
.jpg)
Paras Karmacharya, MD, MS
Vanderbilt University, Nashville, TN
Hermitage, TN, United States
Abstract Poster Presenter(s)
Paras Karmacharya1, Courtney Stull2, Alisa Stephens-Shields3, M. Elaine Husni4, Jose Scher5, Ethan Craig6, Robert Fitzsimmons3, Soumya Reddy7, Marina Magrey8, Alexis Ogdie9 and Jessica Walsh10, 1Vanderbilt University, Nashville, TN, 2University of Pittsburgh, Pittsburgh, PA, 3University of Pennsylvania, Philadelphia, 4Cleveland Clinic, Cleveland, OH, 5New York University School of Medicine, New York, NY, 6University of Pennsylvania, Wallingford, PA, 7NYU School of Medicine, New York, NY, 8Case Western Reserve University, University Hospitals, Richfield, OH, 9Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, 10University of Utah, Salt Lake City, UT
Background/Purpose: Despite significant heterogeneity in psoriatic arthritis (PsA), randomized controlled trials (RCTs) generally enroll a homogenous subgroup of PsA patients (polyarticular similar to rheumatoid arthritis). In clinical practice, however, majority of patients have oligoarticular disease, often making it difficult to observe meaningful changes. To improve our understanding of how therapies work in the "real world" and among understudied patient subgroups, trials in real world environments are crucial. Before conducting pragmatic studies in PsA, however, it is necessary to define the appropriate outcome measures including patient-reported outcomes (PROs). The objectives of this study were to determine the responsiveness to therapy and minimally clinically important improvement (MCII) for PROs in PsA and to examine the impact of baseline disease activity on the ability to demonstrate change.
Methods: A longitudinal cohort study was performed within the PsA Research Consortium (PARC). Patients completed PROs including the Routine Assessment of Patient Index Data, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the PsA Impact of Disease questionnaire, and three PRO Measure Information System (PROMIS) instruments (global 10a, depression 8a, and fatigue 8a). Mean change in the scores between visits and standardized response means (SRMs) were calculated. The MCII was calculated as the mean change in score among patients who reported minimal improvement. SRMs and MCIIs were compared among subgroups with moderate to highly active PsA (≥3 swollen and ≥3 tender joint counts based on 66- and 68- joint counts respectively) and those with less active PsA (< 3 swollen and < 3 tender joint counts). This cut-off is used as an inclusion criterion in most PsA RCTs.
Results: Among 171 patients, 266 therapy courses were included. Mean age was 51 (SD 13.8), 53% were female, and mean body mass index (BMI) was 29.9 (SD 6.5). At baseline, the mean swollen and tender joint counts were 3 and 6, respectively. Therapies initiated included a tumor necrosis factor inhibitor (N=145), interleukin 17 inhibitor (N=55), other biologic or JAK inhibitor (N=14), or an oral small molecule (N=96). SRMs (Figure 1) and MCII for all measures (Table 1 & 2) were small to moderate though greater among those with higher baseline disease activity. BASDAI had the best SRM overall and for less active PsA. cDAPSA and PsAID performed better and comparable to BASDAI respectively in the moderate to highly active PsA subgroup. SRMs were similar among those initiating a biologic therapy compared to those initiating any therapy.
Conclusion: SRMs and MCII were relatively small in this real-world population, particularly among those with lower disease activity at baseline. The PsAID and cDAPSA measures performed better in those with higher disease activity compared to those with lower disease activity. In future pragmatic trials, selection of measures should take into account the projected baseline disease activity of patients enrolled.
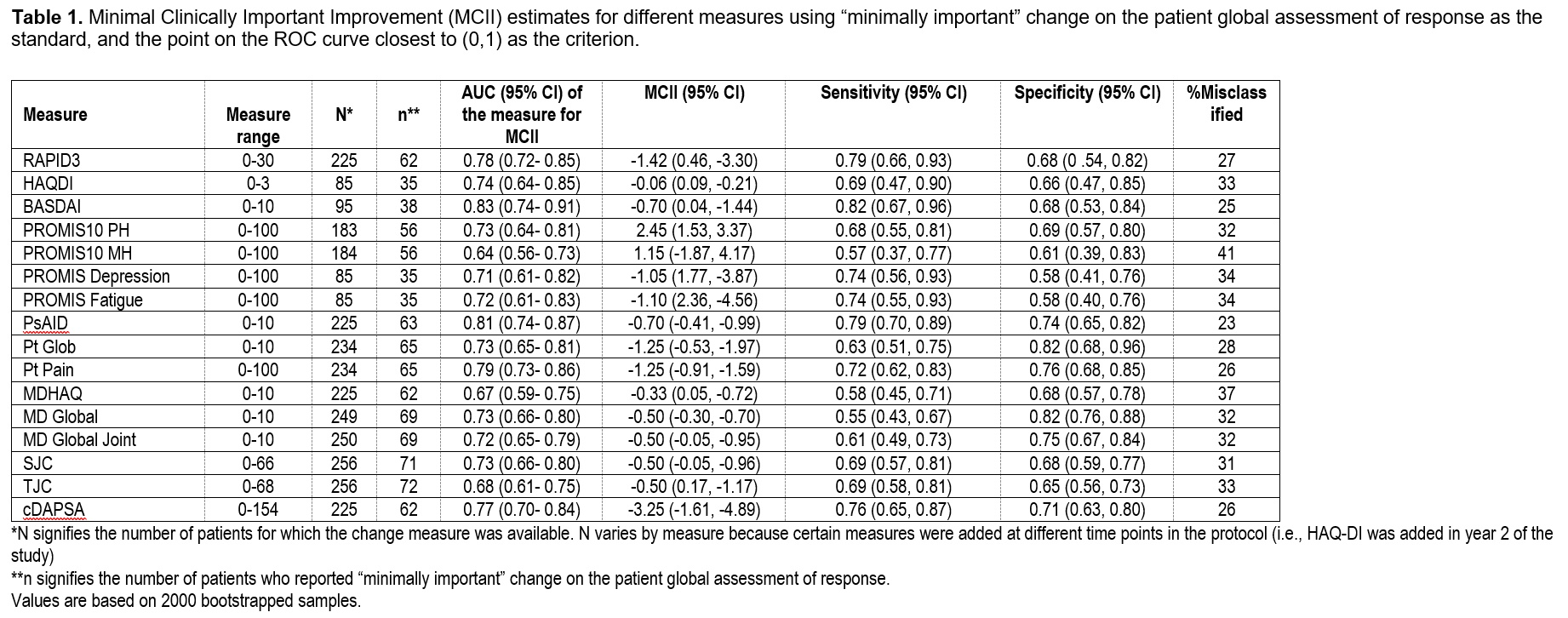 Table 1. Minimal Clinically Important Improvement (MCII) estimates for different measures using “minimally important” change on the patient global assessment of response as the standard, and the point on the ROC curve closest to (0,1) as the criterion.
Table 1. Minimal Clinically Important Improvement (MCII) estimates for different measures using “minimally important” change on the patient global assessment of response as the standard, and the point on the ROC curve closest to (0,1) as the criterion.
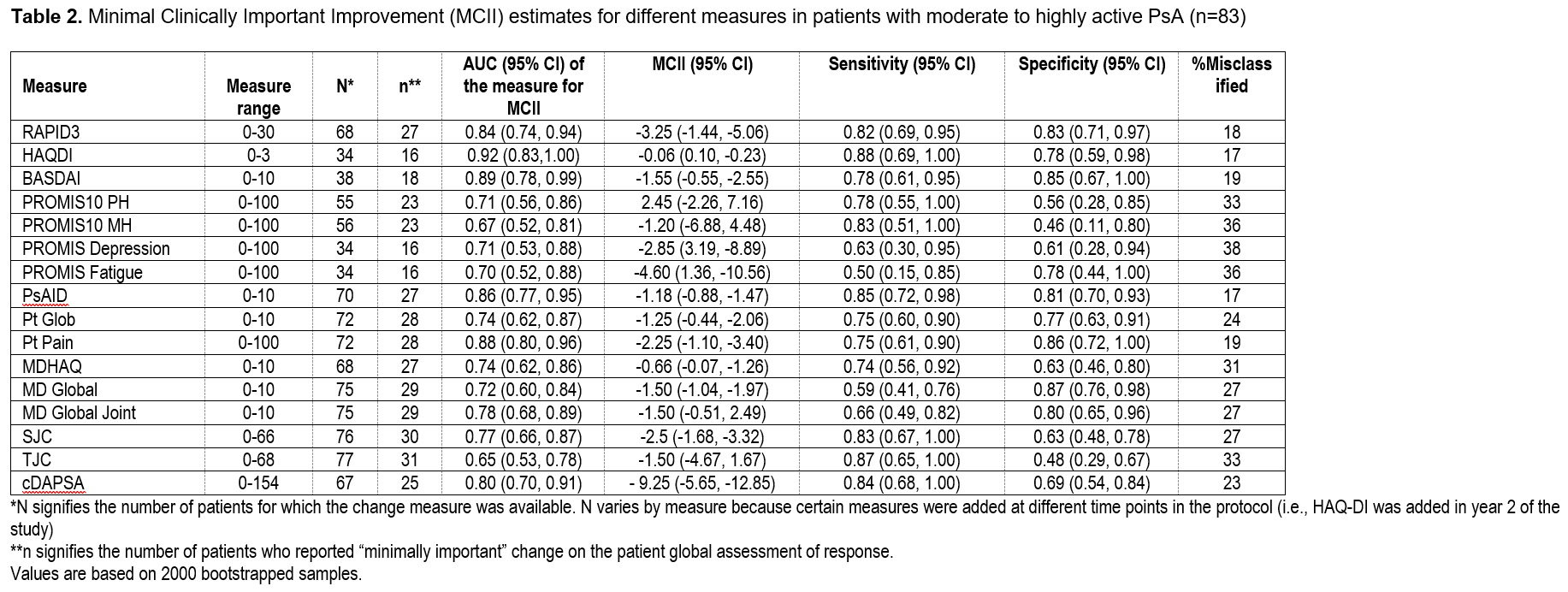 Table 2. Minimal Clinically Important Improvement (MCII) estimates for different measures in patients with moderate to highly active PsA (n=83)
Table 2. Minimal Clinically Important Improvement (MCII) estimates for different measures in patients with moderate to highly active PsA (n=83)
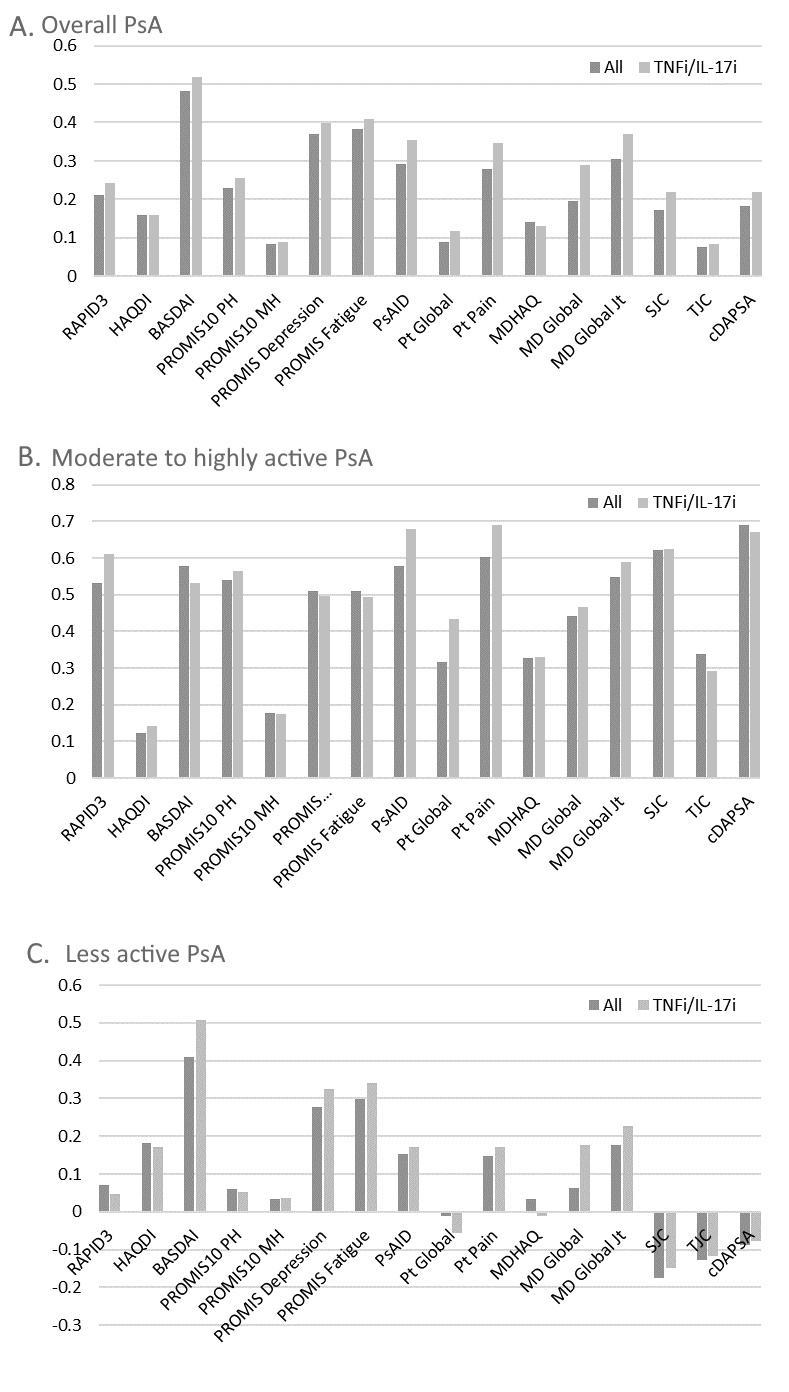 Figure 1. Standard Response Means of different measures tested in PARC.
Figure 1. Standard Response Means of different measures tested in PARC.
Abbreviations: PARC: Psoriatic Arthritis Research Consortium; TNFi: tumor necrosis factor inhibitor; IL17i: interleukin-17 inhibitor; HAQDI: Health Assessment Questionnaire Disability Index; MD global: physician’s global assessment; Pt: patient; SJC: swollen joint count; TJC: tender joint count; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; PSAID: Psoriatic Arthritis Impact of Disease; cDAPSA: clinical Disease Activity of Psoriatic Arthritis; MDHAQ: Multidimensional Health Assessment Questionnaire; PROMIS: Patient-Reported Outcomes Measurement Information System Global Short Form; PROMIS10 MH: Patient-Reported Outcomes Measurement Information System Global Short Form Mental Health; PROMIS10 PH: Patient-Reported Outcomes Measurement Information System Global Short Form Physical Health; RAPID3: Routine Assessment of Patient Index Data.
Disclosures: P. Karmacharya, None; C. Stull, None; A. Stephens-Shields, None; M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; J. Scher, Janssen, Pfizer, AbbVie, Sanofi, Novartis, UCB; E. Craig, None; R. Fitzsimmons, None; S. Reddy, AbbVie/Abbott, Amgen, Novartis, Janssen, Pfizer; M. Magrey, AbbVie, Eli Lilly, Novartis, Pfizer Inc, UCB; A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; J. Walsh, AbbVie, Amgen, Lilly, Novartis, Pfizer, UCB, Celgene, Janssen, Merck.
Background/Purpose: Despite significant heterogeneity in psoriatic arthritis (PsA), randomized controlled trials (RCTs) generally enroll a homogenous subgroup of PsA patients (polyarticular similar to rheumatoid arthritis). In clinical practice, however, majority of patients have oligoarticular disease, often making it difficult to observe meaningful changes. To improve our understanding of how therapies work in the "real world" and among understudied patient subgroups, trials in real world environments are crucial. Before conducting pragmatic studies in PsA, however, it is necessary to define the appropriate outcome measures including patient-reported outcomes (PROs). The objectives of this study were to determine the responsiveness to therapy and minimally clinically important improvement (MCII) for PROs in PsA and to examine the impact of baseline disease activity on the ability to demonstrate change.
Methods: A longitudinal cohort study was performed within the PsA Research Consortium (PARC). Patients completed PROs including the Routine Assessment of Patient Index Data, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the PsA Impact of Disease questionnaire, and three PRO Measure Information System (PROMIS) instruments (global 10a, depression 8a, and fatigue 8a). Mean change in the scores between visits and standardized response means (SRMs) were calculated. The MCII was calculated as the mean change in score among patients who reported minimal improvement. SRMs and MCIIs were compared among subgroups with moderate to highly active PsA (≥3 swollen and ≥3 tender joint counts based on 66- and 68- joint counts respectively) and those with less active PsA (< 3 swollen and < 3 tender joint counts). This cut-off is used as an inclusion criterion in most PsA RCTs.
Results: Among 171 patients, 266 therapy courses were included. Mean age was 51 (SD 13.8), 53% were female, and mean body mass index (BMI) was 29.9 (SD 6.5). At baseline, the mean swollen and tender joint counts were 3 and 6, respectively. Therapies initiated included a tumor necrosis factor inhibitor (N=145), interleukin 17 inhibitor (N=55), other biologic or JAK inhibitor (N=14), or an oral small molecule (N=96). SRMs (Figure 1) and MCII for all measures (Table 1 & 2) were small to moderate though greater among those with higher baseline disease activity. BASDAI had the best SRM overall and for less active PsA. cDAPSA and PsAID performed better and comparable to BASDAI respectively in the moderate to highly active PsA subgroup. SRMs were similar among those initiating a biologic therapy compared to those initiating any therapy.
Conclusion: SRMs and MCII were relatively small in this real-world population, particularly among those with lower disease activity at baseline. The PsAID and cDAPSA measures performed better in those with higher disease activity compared to those with lower disease activity. In future pragmatic trials, selection of measures should take into account the projected baseline disease activity of patients enrolled.
 Table 1. Minimal Clinically Important Improvement (MCII) estimates for different measures using “minimally important” change on the patient global assessment of response as the standard, and the point on the ROC curve closest to (0,1) as the criterion.
Table 1. Minimal Clinically Important Improvement (MCII) estimates for different measures using “minimally important” change on the patient global assessment of response as the standard, and the point on the ROC curve closest to (0,1) as the criterion.  Table 2. Minimal Clinically Important Improvement (MCII) estimates for different measures in patients with moderate to highly active PsA (n=83)
Table 2. Minimal Clinically Important Improvement (MCII) estimates for different measures in patients with moderate to highly active PsA (n=83) Figure 1. Standard Response Means of different measures tested in PARC.
Figure 1. Standard Response Means of different measures tested in PARC.Abbreviations: PARC: Psoriatic Arthritis Research Consortium; TNFi: tumor necrosis factor inhibitor; IL17i: interleukin-17 inhibitor; HAQDI: Health Assessment Questionnaire Disability Index; MD global: physician’s global assessment; Pt: patient; SJC: swollen joint count; TJC: tender joint count; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; PSAID: Psoriatic Arthritis Impact of Disease; cDAPSA: clinical Disease Activity of Psoriatic Arthritis; MDHAQ: Multidimensional Health Assessment Questionnaire; PROMIS: Patient-Reported Outcomes Measurement Information System Global Short Form; PROMIS10 MH: Patient-Reported Outcomes Measurement Information System Global Short Form Mental Health; PROMIS10 PH: Patient-Reported Outcomes Measurement Information System Global Short Form Physical Health; RAPID3: Routine Assessment of Patient Index Data.
Disclosures: P. Karmacharya, None; C. Stull, None; A. Stephens-Shields, None; M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; J. Scher, Janssen, Pfizer, AbbVie, Sanofi, Novartis, UCB; E. Craig, None; R. Fitzsimmons, None; S. Reddy, AbbVie/Abbott, Amgen, Novartis, Janssen, Pfizer; M. Magrey, AbbVie, Eli Lilly, Novartis, Pfizer Inc, UCB; A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; J. Walsh, AbbVie, Amgen, Lilly, Novartis, Pfizer, UCB, Celgene, Janssen, Merck.

