Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0913–0938) RA – Treatment Poster II
0927: Review of 167,990 Same Day RAPID3, CDAI Scores for 61,312 Patients Receiving DMARD Treatment for Rheumatoid Arthritis; Evaluations of Disease Assessments and Treatment Discontinuation
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- NS
Nehad Soloman, MD
Arizona Arthritis & Rheumatology Associates, P.C.
Peoria, AZ, United States
Abstract Poster Presenter(s)
Nehad Soloman1, Kent Kwas Huston2, Jasvinder singh3, Simon Helfgott4, Andrew Frick5, Scott Milligan5 and Colin Edgerton6, 1Arizona Arthritis & Rheumatology Associates, P.C., Peoria, AZ, 2Kansas City Physician Partners Center for Rheumatic Disease, Kansas City, MO, 3University of Alabama at Birmingham, Birmingham, AL, 4Brigham and Women's Hospital, Harvard Medical School, Boston, MA, 5Trio Health, Louisville, CO, 6Articularis Healthcare, Sullivans Island, SC
Background/Purpose: Treatment efficacy for rheumatoid arthritis is often measured through disease assessments containing subjective input from the provider, the patient, or both. Here we compare scores from same-day CDAI and RAPID3 during treatment and at treatment discontinuation, to evaluate whether these disease assessments are aligned and are indicative of disease management.
Methods: Data are specific to patients in care by the American Rheumatology Network, and reside in PIONEER Rheumatology, an enhanced database combining fielded EMR data with extracted information from open text (office visits, infusions logs, and provider-patient communications). Study population: 61,312 patients with RA receiving conventional synthetic, targeted synthetic (ts), and/or biologic (b) DMARDs between 2014 to 2021 and with same day CDAI and RAPID3 scores. Total observations: 167,990 disease assessment scores, 83,995 patient-dates. Analyses conducted using R v.3.6: Spearman's rho (score classifications), Pearson correlation (numeric scores), and Cohen's weighted Kappa (score classification). Discontinuation analyses were limited to ts/bDMARD episodes and scores considered were closest to but between -90 to +14 days of discontinuation. Rapid3 and CDAI were split into low/remission, moderate, and severe disease activity.
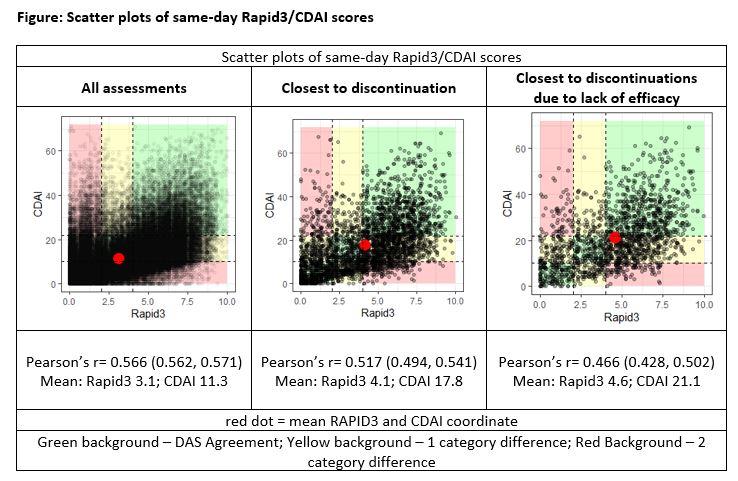
Results: A moderate agreement between same day CDAI and RAPID3 scores was suggested by Cohen's Weighted Kappa (0.42) and Spearman's rho (0.49). By disease severity classifications, a greater proportion of RAPID3 results indicated severe disease (35.8%; 30,057/83,995) v. CDAI (13.8%; 11,618/83,995; p< 0.001). Of the severe results by RAPID3, 25.6% (7683/30,057) were recorded as near-remission/low activity by CDAI. Conversely, 15.6% (1,818/11,618) of CDAI results indicating severe disease were classified as near-remission/low by RAPID3. In subset analyses examining scores at ts/bDMARD discontinuation (n=3600 drug episodes), 53.9% (1,939/3,600) had an accompanying RAPID3 result indicating severe disease compared to 31.6% by CDAI (1,136/3,600; p< 0.001). Of the 3,600 episodes, 47% (1,692) discontinued due to lack or loss of efficacy, as documented in visit notes; the remainder discontinued for non-clinical reasons and/or conditions arising from treatment, disease, or existing patient burden. For ts/bDMARD episodes discontinued for efficacy reasons, 19.6% (332/1,692) had an accompanying RAPID3 result indicating near-remission/low activity and 21.1% (356/1,692) indicated near-remission/low activity by CDAI (p=0.273). Scatter plots of individual scores are provided in the FIGURE.
Conclusion: A moderate correlation was observed between RAPID3, a patient-based disease assessment, and CDAI, a predominantly physician-based disease assessment. However, considerable discord was observed for ~10% of assessments, calling into question which assessment is a better indicator of disease management. Moreover, 20% of ts/bDMARD episodes that discontinued due to lack or loss of efficacy had near-remission/low disease activity by either RAPID3 or CDAI. These data suggest that some patient-important outcomes are not reflected in these assessments.
Disclosures: N. Soloman, AbbVie/Abbott, Horizon, Janssen, Amgen, GlaxoSmithKlein(GSK), AstraZeneca; K. Huston, None; J. singh, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two Labs Inc., Adept Field Solutions, Clinical Care Options, Clearview Healthcare Partners, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, Practice Point Communications, National Institutes of Health, American College of Rheumatology, Zimmer Biomet Holdings, Intuitive Surgical Inc./Philips Electronics North America, TPT Global Tech, Vaxart Pharmaceuticals, Atyu Biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals Holding Corp, Charlotte's Web Holdings, Inc., Amarin, Viking, Moderna Pharmaceuticals, Simply Speaking, Outcomes Measures in Rheumatology (OMERACT), FDA Arthritis Advisory Committee, Veterans Affairs Rheumatology Field Advisory Board (FAB), University of Alabama at Birmingham (UAB) Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; S. Helfgott, Abbott Ltd., Genentech; A. Frick, Trio Health Analytics; S. Milligan, AbbVie/Abbott, AstraZeneca, GlaxoSmithKlein(GSK), UCB; C. Edgerton, Novartis, Boehringer-Ingelheim.
Background/Purpose: Treatment efficacy for rheumatoid arthritis is often measured through disease assessments containing subjective input from the provider, the patient, or both. Here we compare scores from same-day CDAI and RAPID3 during treatment and at treatment discontinuation, to evaluate whether these disease assessments are aligned and are indicative of disease management.
Methods: Data are specific to patients in care by the American Rheumatology Network, and reside in PIONEER Rheumatology, an enhanced database combining fielded EMR data with extracted information from open text (office visits, infusions logs, and provider-patient communications). Study population: 61,312 patients with RA receiving conventional synthetic, targeted synthetic (ts), and/or biologic (b) DMARDs between 2014 to 2021 and with same day CDAI and RAPID3 scores. Total observations: 167,990 disease assessment scores, 83,995 patient-dates. Analyses conducted using R v.3.6: Spearman's rho (score classifications), Pearson correlation (numeric scores), and Cohen's weighted Kappa (score classification). Discontinuation analyses were limited to ts/bDMARD episodes and scores considered were closest to but between -90 to +14 days of discontinuation. Rapid3 and CDAI were split into low/remission, moderate, and severe disease activity.
Results: A moderate agreement between same day CDAI and RAPID3 scores was suggested by Cohen's Weighted Kappa (0.42) and Spearman's rho (0.49). By disease severity classifications, a greater proportion of RAPID3 results indicated severe disease (35.8%; 30,057/83,995) v. CDAI (13.8%; 11,618/83,995; p< 0.001). Of the severe results by RAPID3, 25.6% (7683/30,057) were recorded as near-remission/low activity by CDAI. Conversely, 15.6% (1,818/11,618) of CDAI results indicating severe disease were classified as near-remission/low by RAPID3. In subset analyses examining scores at ts/bDMARD discontinuation (n=3600 drug episodes), 53.9% (1,939/3,600) had an accompanying RAPID3 result indicating severe disease compared to 31.6% by CDAI (1,136/3,600; p< 0.001). Of the 3,600 episodes, 47% (1,692) discontinued due to lack or loss of efficacy, as documented in visit notes; the remainder discontinued for non-clinical reasons and/or conditions arising from treatment, disease, or existing patient burden. For ts/bDMARD episodes discontinued for efficacy reasons, 19.6% (332/1,692) had an accompanying RAPID3 result indicating near-remission/low activity and 21.1% (356/1,692) indicated near-remission/low activity by CDAI (p=0.273). Scatter plots of individual scores are provided in the FIGURE.
Conclusion: A moderate correlation was observed between RAPID3, a patient-based disease assessment, and CDAI, a predominantly physician-based disease assessment. However, considerable discord was observed for ~10% of assessments, calling into question which assessment is a better indicator of disease management. Moreover, 20% of ts/bDMARD episodes that discontinued due to lack or loss of efficacy had near-remission/low disease activity by either RAPID3 or CDAI. These data suggest that some patient-important outcomes are not reflected in these assessments.

Disclosures: N. Soloman, AbbVie/Abbott, Horizon, Janssen, Amgen, GlaxoSmithKlein(GSK), AstraZeneca; K. Huston, None; J. singh, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two Labs Inc., Adept Field Solutions, Clinical Care Options, Clearview Healthcare Partners, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, Practice Point Communications, National Institutes of Health, American College of Rheumatology, Zimmer Biomet Holdings, Intuitive Surgical Inc./Philips Electronics North America, TPT Global Tech, Vaxart Pharmaceuticals, Atyu Biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals Holding Corp, Charlotte's Web Holdings, Inc., Amarin, Viking, Moderna Pharmaceuticals, Simply Speaking, Outcomes Measures in Rheumatology (OMERACT), FDA Arthritis Advisory Committee, Veterans Affairs Rheumatology Field Advisory Board (FAB), University of Alabama at Birmingham (UAB) Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; S. Helfgott, Abbott Ltd., Genentech; A. Frick, Trio Health Analytics; S. Milligan, AbbVie/Abbott, AstraZeneca, GlaxoSmithKlein(GSK), UCB; C. Edgerton, Novartis, Boehringer-Ingelheim.

