Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0695–0723) Epidemiology and Public Health Poster I
0720: Risk-Benefit Assessment of Primary Prophylaxis for Pneumocystis Pneumonia in Patents with Rheumatic Diseases Exposed to Rituximab: A Multicenter Cohort Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- JP
Jun Won Park, MD
Seoul National University Hospital
Seoul, South Korea
Abstract Poster Presenter(s)
Jun Won Park1, Jeffrey Curtis2, Min Jung Kim3, You-Jung Ha4, Yun Jong Lee4 and Eun Bong Lee1, 1Seoul National University Hospital, Seoul, Republic of Korea, 2Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, Birmingham, AL, 3Seoul Metropolitan Government Boramae Medical center, Dongjak-gu, Seoul, Republic of Korea, 4Seoul National University Bundang Hospital, Seongnam, Republic of Korea
Background/Purpose: Although previous studies suggested that primary prophylaxis for pneumocystis pneumonia (PJP) during rituximab treatment could be beneficial, it is uncertain whether this strategy should be universally performed in patients with rheumatic diseases. In this study, we investigated the risk factors for PJP in patients with rheumatic diseases exposed to rituximab to establish a specific indication necessitating primary PJP prophylaxis during treatment.
Methods: This cohort study included 818 patients treated with rituximab for rheumatic diseases from 3 medical institutions in South Korea. Patients were classified into a control group (n = 399) and a prophylaxis group (n = 419) according to the administration of prophylactic trimethoprim-sulfamethoxazole (TMP-SMX) during the first 28 days from the initiation of rituximab (defined as lead-in period). Primary outcome of this study was 1-year incidence of PJP in the two groups. Incidence rates of adverse events in the two groups and adverse drug reactions (ADRs) associated with TMP-SMX were also investigated. To minimize confounding by indication occurred in selecting patients with prophylaxis, the stabilized inverse probability of treatment weights (sIPTW) were used.
Results: The baseline characteristics of the control and the prophylaxis groups are presented in Table 1. Proportion of the patients with concomitant high-dose glucocorticoid treatment (≥30mg/day of prednisone or equivalent) during the lead-in period was significantly higher in the prophylaxis group (24.1% vs. 63.5%). After applying sIPTW, all measured covariates were all balanced.
During a total of 663.1 person-years of observation, 11 PJP infections occurred, with a related mortality of 45.5% (5/11). One-year incidence of PJP (per 100 person-year) was 2.16 (95% CI 0.87-4.46) in the control group and 1.18 (0.03-3.02) in the prophylaxis group. However, 3 out of 4 PJP in the prophylaxis group occurred after discontinuation of prophylactic TMP-SMX.
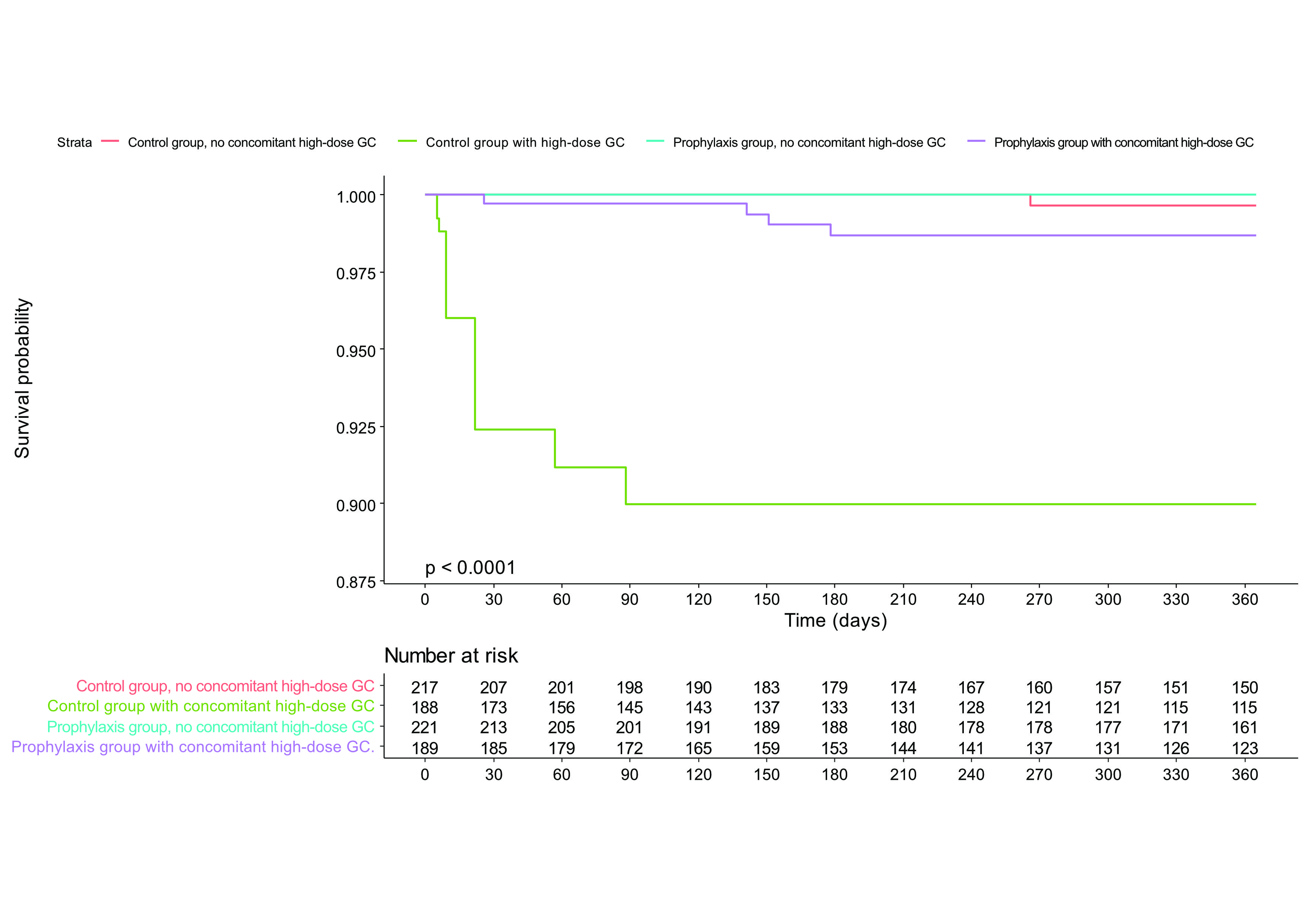
Concomitant high-dose glucocorticoid treatment during the lead-in period was the most important risk factor for PJP (HR = 40.65, 95% CI 4.81-343.40) and only one PJP case occurred in patients not exposed to it. Prophylactic effect of TMP-SMX was different according to whether a patients received concomitant high-dose glucocorticoid. TMP-SMX significantly decreased the PJP incidence only in the subgroup of concomitant high-dose glucocorticoid (adjusted HR 0.11 [0.03-0.37]) (Figure). The frequency of adverse events was comparable between the two groups and only 2 severe ADRs (one aminotransferase increase and one pancytopenia) related to TMP-SMX occurred. The number needed to treat (NNT) to prevent one case of PJP in patients with concomitant high-dose glucocorticoid was 26 (95% CI 21.95-34.80) whereas the number needed to harm (NNH) for severe ADR was 210 (87.9-∞).
Conclusion: Primary prophylaxis for PJP using TMP-SMX is beneficial when a rituximab treatment is combined with high-dose glucocorticoid treatment.
.jpg)
Disclosures: J. Park, None; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; M. Kim, None; Y. Ha, None; Y. Lee, None; E. Lee, None.
Background/Purpose: Although previous studies suggested that primary prophylaxis for pneumocystis pneumonia (PJP) during rituximab treatment could be beneficial, it is uncertain whether this strategy should be universally performed in patients with rheumatic diseases. In this study, we investigated the risk factors for PJP in patients with rheumatic diseases exposed to rituximab to establish a specific indication necessitating primary PJP prophylaxis during treatment.
Methods: This cohort study included 818 patients treated with rituximab for rheumatic diseases from 3 medical institutions in South Korea. Patients were classified into a control group (n = 399) and a prophylaxis group (n = 419) according to the administration of prophylactic trimethoprim-sulfamethoxazole (TMP-SMX) during the first 28 days from the initiation of rituximab (defined as lead-in period). Primary outcome of this study was 1-year incidence of PJP in the two groups. Incidence rates of adverse events in the two groups and adverse drug reactions (ADRs) associated with TMP-SMX were also investigated. To minimize confounding by indication occurred in selecting patients with prophylaxis, the stabilized inverse probability of treatment weights (sIPTW) were used.
Results: The baseline characteristics of the control and the prophylaxis groups are presented in Table 1. Proportion of the patients with concomitant high-dose glucocorticoid treatment (≥30mg/day of prednisone or equivalent) during the lead-in period was significantly higher in the prophylaxis group (24.1% vs. 63.5%). After applying sIPTW, all measured covariates were all balanced.
During a total of 663.1 person-years of observation, 11 PJP infections occurred, with a related mortality of 45.5% (5/11). One-year incidence of PJP (per 100 person-year) was 2.16 (95% CI 0.87-4.46) in the control group and 1.18 (0.03-3.02) in the prophylaxis group. However, 3 out of 4 PJP in the prophylaxis group occurred after discontinuation of prophylactic TMP-SMX.
Concomitant high-dose glucocorticoid treatment during the lead-in period was the most important risk factor for PJP (HR = 40.65, 95% CI 4.81-343.40) and only one PJP case occurred in patients not exposed to it. Prophylactic effect of TMP-SMX was different according to whether a patients received concomitant high-dose glucocorticoid. TMP-SMX significantly decreased the PJP incidence only in the subgroup of concomitant high-dose glucocorticoid (adjusted HR 0.11 [0.03-0.37]) (Figure). The frequency of adverse events was comparable between the two groups and only 2 severe ADRs (one aminotransferase increase and one pancytopenia) related to TMP-SMX occurred. The number needed to treat (NNT) to prevent one case of PJP in patients with concomitant high-dose glucocorticoid was 26 (95% CI 21.95-34.80) whereas the number needed to harm (NNH) for severe ADR was 210 (87.9-∞).
Conclusion: Primary prophylaxis for PJP using TMP-SMX is beneficial when a rituximab treatment is combined with high-dose glucocorticoid treatment.
.jpg)

Disclosures: J. Park, None; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; M. Kim, None; Y. Ha, None; Y. Lee, None; E. Lee, None.

