Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0913–0938) RA – Treatment Poster II
0931: Rituximab versus Leflunomide in Combination with Methotrexate in Patients with Active Rheumatoid Arthritis Despite Methotrexate Treatment- an Open Label Randomized Controlled Trial
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- RR
Rashmi Roongta, MD, MBBS, DM
Institute of Postgraduate Medical Education and Research
Kolkata, West Bengal, India
Abstract Poster Presenter(s)
Rashmi Roongta1, Geetabali Sircar1, Parasar Ghosh2, Dipendranath Ghosh1 and Hiramanik Sit1, 1Institute of Postgraduate Medical Education and Research, Kolkata, West Bengal, India, 2Govt of West Bengal, Kolkata, India, Kolkata, West Bengal, India
Background/Purpose: Active rheumatoid arthritis (RA) despite methotrexate (MTX) monotherapy is seen in about 40-50% of patients1. The current ACR guidelines recommend adding a biologic or targeted synthetic Disease Modifying Anti-Rheumatic Drug (b/ts DMARD) to MTX therapy in such cases. The European Alliance of Associations for Rheumatology (EULAR) guidelines recommend a combination of DMARDs but the most preferred combination is that of MTX with sulphasalazine and hydroxychloroquine. In a resource-limited country like ours, it is not possible to add a bDMARD to every patient who fails MTX therapy, and a more cost-effective method needs to be implemented. Often, leflunomide (LEF) is added in such cases and is effective. We aimed to study if LEF with MTX combination is non-inferior to rituximab (RTX) and MTX combination in patients with active RA despite MTX.
Methods: This open-label, randomized controlled trial, was carried out on adult patients with RA having moderate to high disease activity [defined by Disease Activity Score 28 (DAS28)] despite MTX therapy for at least 3 months (Clinical Trials Registry- India - REF/2021/04/042755). It was conducted in a tertiary care center in India. Patients were randomized to receive either LEF (10-20 mg/day) or RTX (2 doses of 1gm each, 2 weeks apart) along with background MTX (10-25mg/week). Other conventional synthetic DMARDs were stopped. In patients on oral steroids, an attempt to taper them was made. The participants were followed up at 12, 18, and 24 weeks. The primary outcome was ACR20 response at 24 weeks and secondary outcomes were ACR50 and 70 responses at 24 weeks.
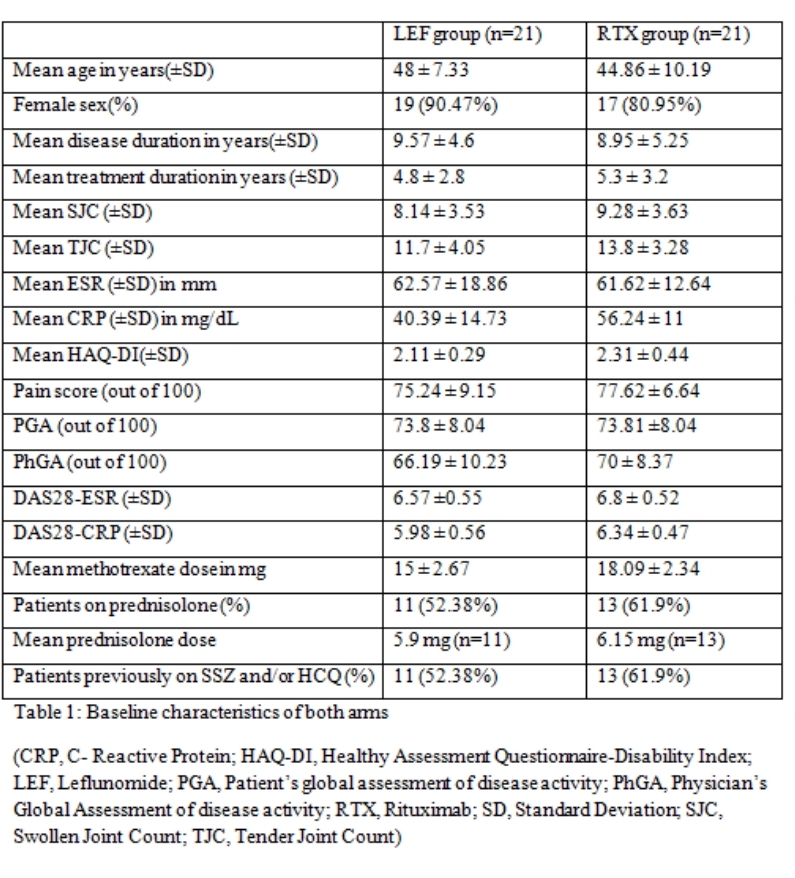
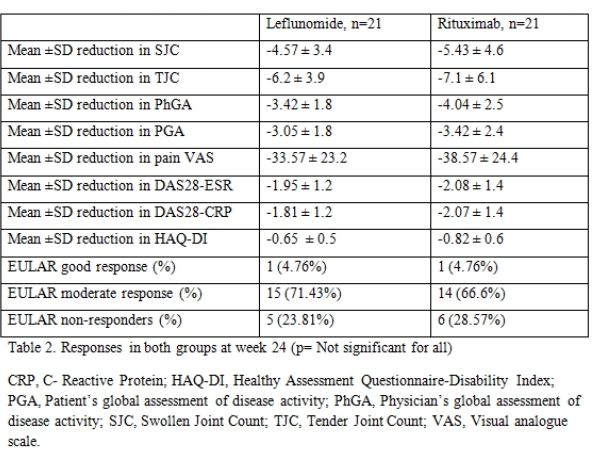
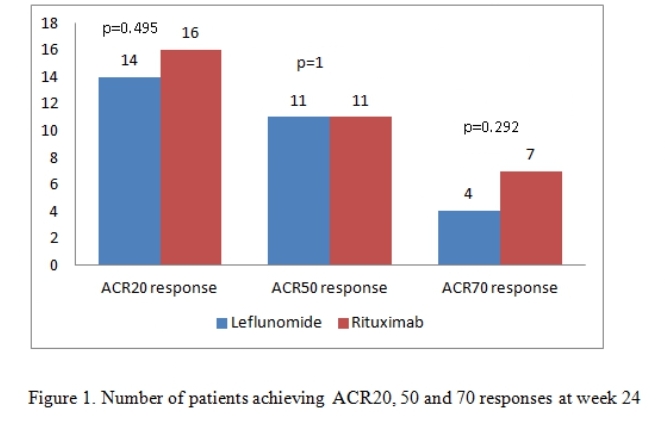
Results: There were 21 patients in each arm. The baseline characteristics were similar in both groups (Table 1) except for a higher mean C-Reactive Protein (CRP) in the RTX arm. The mean ± SD DAS28-CRP scores in the LEF and RTX arms were 5.98 ± 0.56 and 6.34 ± 0.47 respectively and the DAS28-ESR scores were 6.57 ±0.55 and 6.8 ± 0.52 respectively (Table 2). The ACR20 response at 24 weeks was achieved by 14 (66.6%) and 16 (76.19%) patients respectively in the leflunomide and rituximab arms (p=0.734). The ACR50 response was achieved by 11 (52.38%) patients in both groups and ACR70 response by 4 (19%) and 7 (33.3%) in the leflunomide and rituximab arms (p= NS in both) (Figure 1). Four patients in both groups could discontinue prednisolone. Two patients in the RTX group had infusion reactions during the first dose which were managed with anti-histamines and steroids. No infections were reported during the study period in any group. Mild transaminitis was noted in four patients receiving LEF but none required drug withdrawal (< 3 times upper limit of normal). The transaminitis was more frequent in patients taking 20mg LEF per day.
Conclusion: There was no significant difference in the primary or secondary outcomes between both groups. LEF was non-inferior to RTX for the achievement of ACR20/50/70 responses at 24 weeks. LEF can be considered as an add-on option to methotrexate instead of more expensive biologic agents in case of methotrexate refractory RA. Larger studies are needed to confirm this hypothesis.
Disclosures: R. Roongta, None; G. Sircar, None; P. Ghosh, None; D. Ghosh, None; H. Sit, None.
Background/Purpose: Active rheumatoid arthritis (RA) despite methotrexate (MTX) monotherapy is seen in about 40-50% of patients1. The current ACR guidelines recommend adding a biologic or targeted synthetic Disease Modifying Anti-Rheumatic Drug (b/ts DMARD) to MTX therapy in such cases. The European Alliance of Associations for Rheumatology (EULAR) guidelines recommend a combination of DMARDs but the most preferred combination is that of MTX with sulphasalazine and hydroxychloroquine. In a resource-limited country like ours, it is not possible to add a bDMARD to every patient who fails MTX therapy, and a more cost-effective method needs to be implemented. Often, leflunomide (LEF) is added in such cases and is effective. We aimed to study if LEF with MTX combination is non-inferior to rituximab (RTX) and MTX combination in patients with active RA despite MTX.
Methods: This open-label, randomized controlled trial, was carried out on adult patients with RA having moderate to high disease activity [defined by Disease Activity Score 28 (DAS28)] despite MTX therapy for at least 3 months (Clinical Trials Registry- India - REF/2021/04/042755). It was conducted in a tertiary care center in India. Patients were randomized to receive either LEF (10-20 mg/day) or RTX (2 doses of 1gm each, 2 weeks apart) along with background MTX (10-25mg/week). Other conventional synthetic DMARDs were stopped. In patients on oral steroids, an attempt to taper them was made. The participants were followed up at 12, 18, and 24 weeks. The primary outcome was ACR20 response at 24 weeks and secondary outcomes were ACR50 and 70 responses at 24 weeks.
Results: There were 21 patients in each arm. The baseline characteristics were similar in both groups (Table 1) except for a higher mean C-Reactive Protein (CRP) in the RTX arm. The mean ± SD DAS28-CRP scores in the LEF and RTX arms were 5.98 ± 0.56 and 6.34 ± 0.47 respectively and the DAS28-ESR scores were 6.57 ±0.55 and 6.8 ± 0.52 respectively (Table 2). The ACR20 response at 24 weeks was achieved by 14 (66.6%) and 16 (76.19%) patients respectively in the leflunomide and rituximab arms (p=0.734). The ACR50 response was achieved by 11 (52.38%) patients in both groups and ACR70 response by 4 (19%) and 7 (33.3%) in the leflunomide and rituximab arms (p= NS in both) (Figure 1). Four patients in both groups could discontinue prednisolone. Two patients in the RTX group had infusion reactions during the first dose which were managed with anti-histamines and steroids. No infections were reported during the study period in any group. Mild transaminitis was noted in four patients receiving LEF but none required drug withdrawal (< 3 times upper limit of normal). The transaminitis was more frequent in patients taking 20mg LEF per day.
Conclusion: There was no significant difference in the primary or secondary outcomes between both groups. LEF was non-inferior to RTX for the achievement of ACR20/50/70 responses at 24 weeks. LEF can be considered as an add-on option to methotrexate instead of more expensive biologic agents in case of methotrexate refractory RA. Larger studies are needed to confirm this hypothesis.



Disclosures: R. Roongta, None; G. Sircar, None; P. Ghosh, None; D. Ghosh, None; H. Sit, None.

