Back
Poster Session B
Antiphospholipid Syndrome
Session: (0671–0694) Antiphospholipid Syndrome Poster
0684: Single Antiphospholipid Antibody Positive Thrombotic APS Patients’ Clinical Characteristics: Retrospective Results from the APS ACTION Clinical Database and Repository (Registry)
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- SS
Savino Sciascia, MD
University of Turin
Torino, Turin, Italy
Abstract Poster Presenter(s)
Silvia Grazietta Foddai1, Irene Cecchi2, Massimo Radin1, Danieli De Andrade3, Maria Tektonidou4, Vittorio Pengo5, Guillermo Ruiz-Irastorza6, H. Michael Belmont7, Maria Gerosa8, Paul Fortin9, Rosario Lopez-Pedrera10, Zhouli Zhang11, Tatsuya Atsumi12, Guilherme de Jesus13, Hannah Cohen14, Nina Kello15, Ware Branch16, Denis Wahl17, Laura Andreoli18, Esther Rodriguez19, Michelle Petri20, Ann E. Clarke21, Ricard Cervera22, Jason Knight23, Bahar Artim-Esen24, Rohan Willis25, Guillermo Pons-Estel26, Doruk Erkan27, Dario Roccatello1 and Savino Sciascia2, 1University of Turin, Turin, Italy, 2University of Turin, Torino, Italy, 3University of São Paulo, São Paulo, Brazil, 4University of Athens, Athens, Greece, 5University of Padova, Padova, Italy, 6University of the Basque Country, Barakaldo, Spain, 7New York University School of Medicine, New York, NY, 8University of Milan, Milano, Italy, 9CHU de Québec Université Laval, Québec, QC, Canada, 10University of Cordoba, Cordoba, Spain, 11Beijing Medical University, Beijing, China, 12Hokkaido University, Sapporo, Japan, 13Universidade do Estado do Rio de Janeiro, Rio De Janeiro, Brazil, 14University College London, London, United Kingdom, 15Northwell Health, New York, NY, 16University of Utah Health, Salt Lake City, 17Nancy University Hospital, Nancy, France, 18University of Brescia, Brescia, Italy, 19Hospital Universitario 12 de Octubre, Madrid, Spain, 20Johns Hopkins University School of Medicine, Baltimore, MD, 21University of Calgary, Calgary, AB, Canada, 22University of Barcelona, Barcelona, Spain, 23University of Michigan, Ann Arbor, MI, 24Istanbul University, Istanbul, Turkey, 25University of Texas, Galveston, TX, 26Centro Regional de Enfermedades Autoinmunes y Reumáticas, Rosario, Argentina, 27Barbara Volcker Center for Women and Rheumatic Diseases, New York, NY
Background/Purpose: The APS ACTION is a network collecting and analyzing data ofaPL positive patients recruited by international centers. Based on the assumption that triple aPL-positive (LA, aCL IgG/M, and aβ2GPI IgG/M)patients are at higher risk for thrombosis, we investigated the clinical phenotypeof single aPL-positive(LA, aCL IgG/M, oraβ2GPI IgG/M) patients in a large cohort of primary thrombotic APS subjects.
Methods: APS ACTION Registry includes persistently aPL positive patients with or without other systemic autoimmune diseases. We screened the registry for primary thrombotic APS patientswith persistent single aPL positivity (defined as LA, aCL, and aβ2GPI results [from local laboratories] all available at registry entry with two consecutive resultsandonly one aPLtest being persistently positive in accordance to the revised Sapporo Criteria). For each patient, we assessed the site of the event, the type and isotype of aPL, and the number of retrospective documented recurrences. Single aPL-positive patients, with no history of thrombotic or obstetric APS, were used as a control group for comparing the prevalence of different isotypes.Basic statistical analysis was performed using SPSS 26.0.
Results: Of 427 primary APS patientscollected in the registry, 233 triple aPL-tested patients were included in the analysis and63/233(27%) had persistent single aPL positivity (66% women, 77% white): 45(71%) single LA positive, 9 (15%) single aCL(8 IgG,1 IgM), and 9 (15%) single aβ2GPI (7 IgG, 2 IgM). As a comparison, of 66 triple aPL-tested patients with no thrombotic or obstetric APS, 22 hadpersistent single aPL-positivity (68% women, 90% white): nine (41%) single LA positive, eight (36%) single aCL(3 IgG, 5 IgM), and five (23%) single aβ2GPI (2 IgG, 3 IgM). Single LA positivity was significantly more common in the thrombotic APS cohort compared toaPL carriers(71% Vs 41%, p:0.021).
Associations between different aPL tests and clinical manifestations are shown in Table 1. Among single aPL-positive patients with history of arterial or venous thrombosis, 68% and 75% had single LA-positivity, respectively. Based on small numbers, a trend for a higher frequency was observed: a) for arterial thrombosis in single aCL-positive patients(aCL+ 7/9 [78%] Vs. aCL- 2/9 [22%]);and b) for venous thrombosis insingle aB2GPI-positive patients (aβ2GPI+ 8/9 [89%] Vs aβ2GPI- [1/9] 11%).
A history of thrombosis recurrence was observed in 18(28%) patients. However, nosignificant difference was detected between aPL profile and recurrences (both for number and type of events).
Conclusion: Based on the analysis of an international persistently aPL-positive cohort with no other systemic autoimmune diseases: a) approximately 30% of patients have "single" aPL positivity) the majority of "single" aPL-positive patients have LA-positivity (64%), which is more frequent among those with thrombotic APS (71%), compared to those without APS classification (41%); and c) approximately one-third of "single" aPL-positive patients have history of recurrent thrombosis.
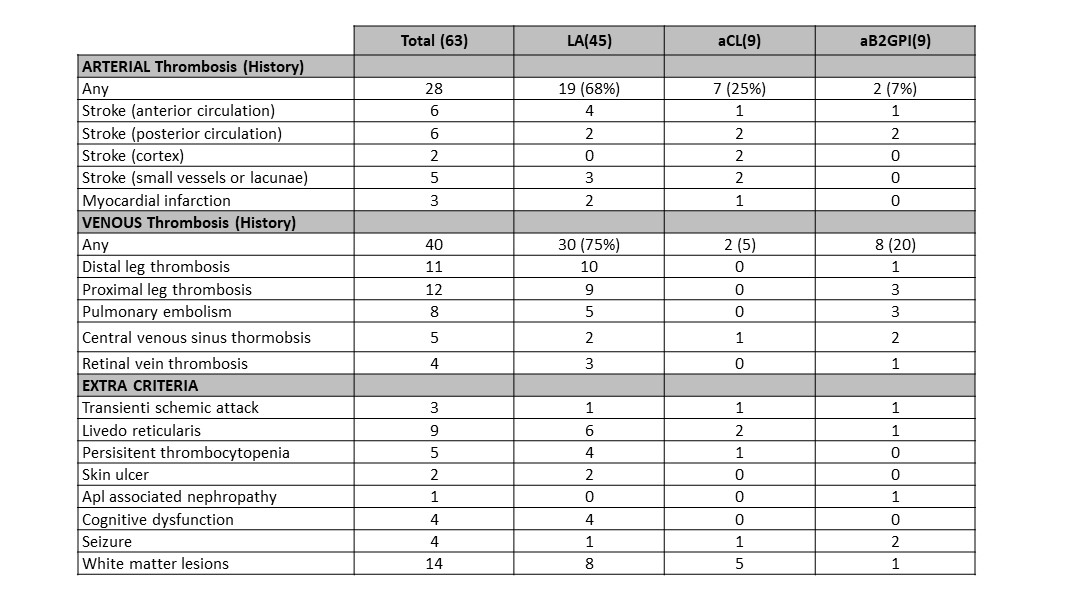 Table 1. Associations between different aPL tests and clinical manifestations.
Table 1. Associations between different aPL tests and clinical manifestations.
Disclosures: S. Foddai, None; I. Cecchi, None; M. Radin, None; D. De Andrade, None; M. Tektonidou, None; V. Pengo, None; G. Ruiz-Irastorza, None; H. Belmont, None; M. Gerosa, None; P. Fortin, None; R. Lopez-Pedrera, None; Z. Zhang, None; T. Atsumi, None; G. de Jesus, None; H. Cohen, None; N. Kello, None; W. Branch, None; D. Wahl, None; L. Andreoli, None; E. Rodriguez, None; M. Petri, None; A. Clarke, None; R. Cervera, None; J. Knight, None; B. Artim-Esen, None; R. Willis, None; G. Pons-Estel, None; D. Erkan, None; D. Roccatello, None; S. Sciascia, None.
Background/Purpose: The APS ACTION is a network collecting and analyzing data ofaPL positive patients recruited by international centers. Based on the assumption that triple aPL-positive (LA, aCL IgG/M, and aβ2GPI IgG/M)patients are at higher risk for thrombosis, we investigated the clinical phenotypeof single aPL-positive(LA, aCL IgG/M, oraβ2GPI IgG/M) patients in a large cohort of primary thrombotic APS subjects.
Methods: APS ACTION Registry includes persistently aPL positive patients with or without other systemic autoimmune diseases. We screened the registry for primary thrombotic APS patientswith persistent single aPL positivity (defined as LA, aCL, and aβ2GPI results [from local laboratories] all available at registry entry with two consecutive resultsandonly one aPLtest being persistently positive in accordance to the revised Sapporo Criteria). For each patient, we assessed the site of the event, the type and isotype of aPL, and the number of retrospective documented recurrences. Single aPL-positive patients, with no history of thrombotic or obstetric APS, were used as a control group for comparing the prevalence of different isotypes.Basic statistical analysis was performed using SPSS 26.0.
Results: Of 427 primary APS patientscollected in the registry, 233 triple aPL-tested patients were included in the analysis and63/233(27%) had persistent single aPL positivity (66% women, 77% white): 45(71%) single LA positive, 9 (15%) single aCL(8 IgG,1 IgM), and 9 (15%) single aβ2GPI (7 IgG, 2 IgM). As a comparison, of 66 triple aPL-tested patients with no thrombotic or obstetric APS, 22 hadpersistent single aPL-positivity (68% women, 90% white): nine (41%) single LA positive, eight (36%) single aCL(3 IgG, 5 IgM), and five (23%) single aβ2GPI (2 IgG, 3 IgM). Single LA positivity was significantly more common in the thrombotic APS cohort compared toaPL carriers(71% Vs 41%, p:0.021).
Associations between different aPL tests and clinical manifestations are shown in Table 1. Among single aPL-positive patients with history of arterial or venous thrombosis, 68% and 75% had single LA-positivity, respectively. Based on small numbers, a trend for a higher frequency was observed: a) for arterial thrombosis in single aCL-positive patients(aCL+ 7/9 [78%] Vs. aCL- 2/9 [22%]);and b) for venous thrombosis insingle aB2GPI-positive patients (aβ2GPI+ 8/9 [89%] Vs aβ2GPI- [1/9] 11%).
A history of thrombosis recurrence was observed in 18(28%) patients. However, nosignificant difference was detected between aPL profile and recurrences (both for number and type of events).
Conclusion: Based on the analysis of an international persistently aPL-positive cohort with no other systemic autoimmune diseases: a) approximately 30% of patients have "single" aPL positivity) the majority of "single" aPL-positive patients have LA-positivity (64%), which is more frequent among those with thrombotic APS (71%), compared to those without APS classification (41%); and c) approximately one-third of "single" aPL-positive patients have history of recurrent thrombosis.
 Table 1. Associations between different aPL tests and clinical manifestations.
Table 1. Associations between different aPL tests and clinical manifestations.Disclosures: S. Foddai, None; I. Cecchi, None; M. Radin, None; D. De Andrade, None; M. Tektonidou, None; V. Pengo, None; G. Ruiz-Irastorza, None; H. Belmont, None; M. Gerosa, None; P. Fortin, None; R. Lopez-Pedrera, None; Z. Zhang, None; T. Atsumi, None; G. de Jesus, None; H. Cohen, None; N. Kello, None; W. Branch, None; D. Wahl, None; L. Andreoli, None; E. Rodriguez, None; M. Petri, None; A. Clarke, None; R. Cervera, None; J. Knight, None; B. Artim-Esen, None; R. Willis, None; G. Pons-Estel, None; D. Erkan, None; D. Roccatello, None; S. Sciascia, None.

