Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0629–0670) SLE – Etiology and Pathogenesis Poster
0629: The Cellular Metabolism of SLE NK Cells Is Primarily Altered at the Level of Mitochondrial Respiration
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- NF
Natalia Fluder, MSc
University Hospital and University of Lausanne
Epalinges, Switzerland
Abstract Poster Presenter(s)
Natalia Fluder, Morgane Humbel, camillo Ribi and Denis Comte, Service of Immunology and Allergy / CHUV, Lausanne, Switzerland
Background/Purpose: Systemic lupus erythematosus (SLE) is a systemic inflammatory disorder, which involves a loss of tolerance and development of autoantibodies. The role of Natural Killer (NK) cells in SLE remains poorly understood. Studies have shown that SLE NK cells are decreased in the peripheral blood, exhibit reduced cytotoxicity, and impaired cytokine production. To date, few studies have explored the molecular mechanisms underlying NK cell dysfunction in SLE.
Herein, we examined metabolic alterations of human SLE NK cells, compared to healthy controls. First, we characterized the glycolysis and oxidative phosphorylation (OXPHOS). Then, we examined the mitochondrial fitness of SLE NK cells.
Methods: SLE and healthy NK cells were isolated from peripheral blood mononuclear cells (PBMC). Glycolysis and OXPHOS were assessed by measuring the extracellular acidification rate (ECAR) and the oxidative consumption rate (OCR), respectively, using the XFe96 Seahorse. Mitochondrial function (membrane potential, mitochondrial mass), mitochondrial superoxide levels and acidification of lysosomes were assessed by flow cytometry. In parallel, mitochondrial membrane potential and mass were observed and quantified, by confocal microscopy. Further, mitochondrial ultrastructure was investigated by transmission electron microscopy. Finally, the ratio of mitochondrial DNA over nuclear DNA was measured using quantitative polymerase chain reaction (qPCR).
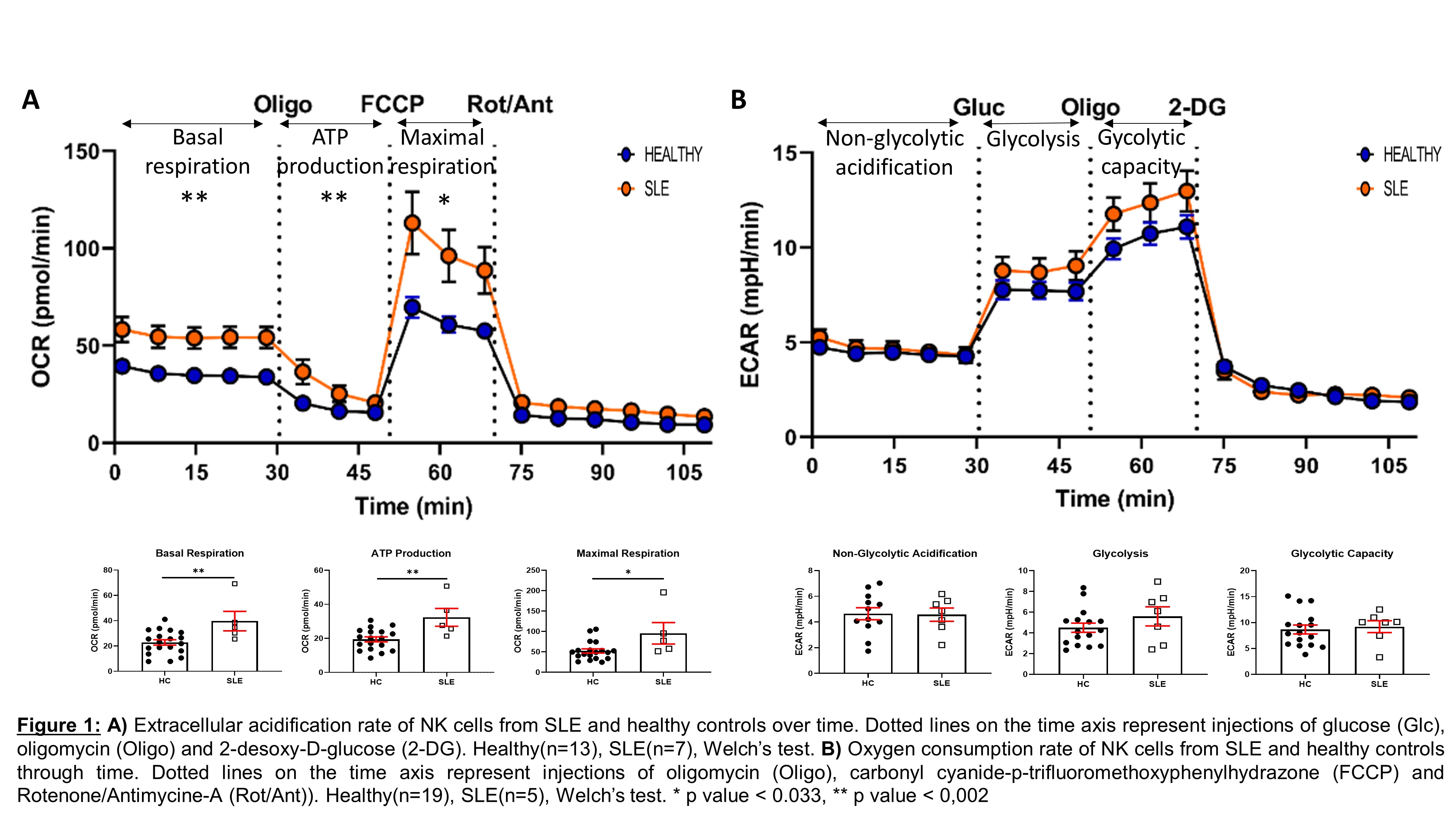
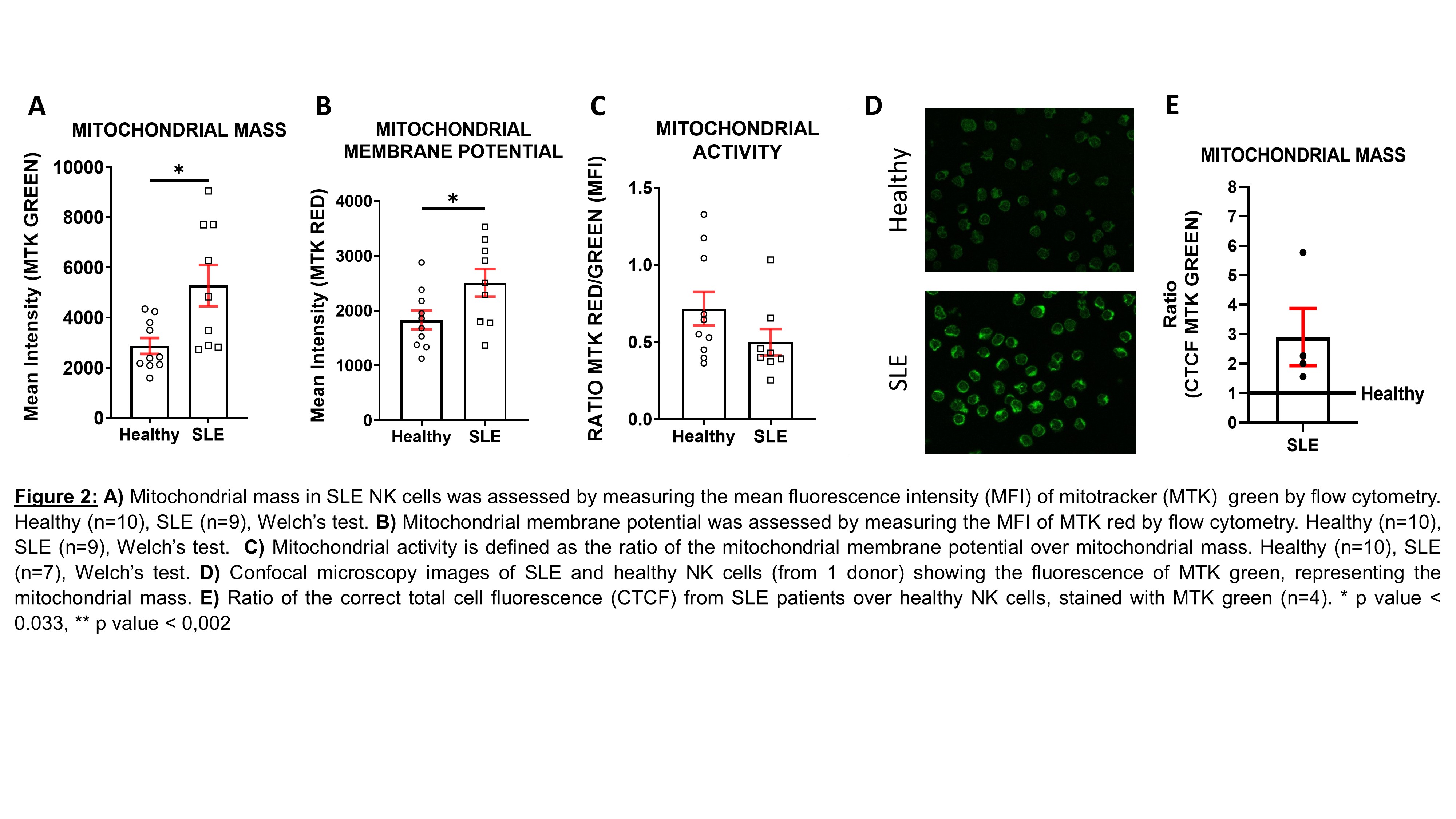
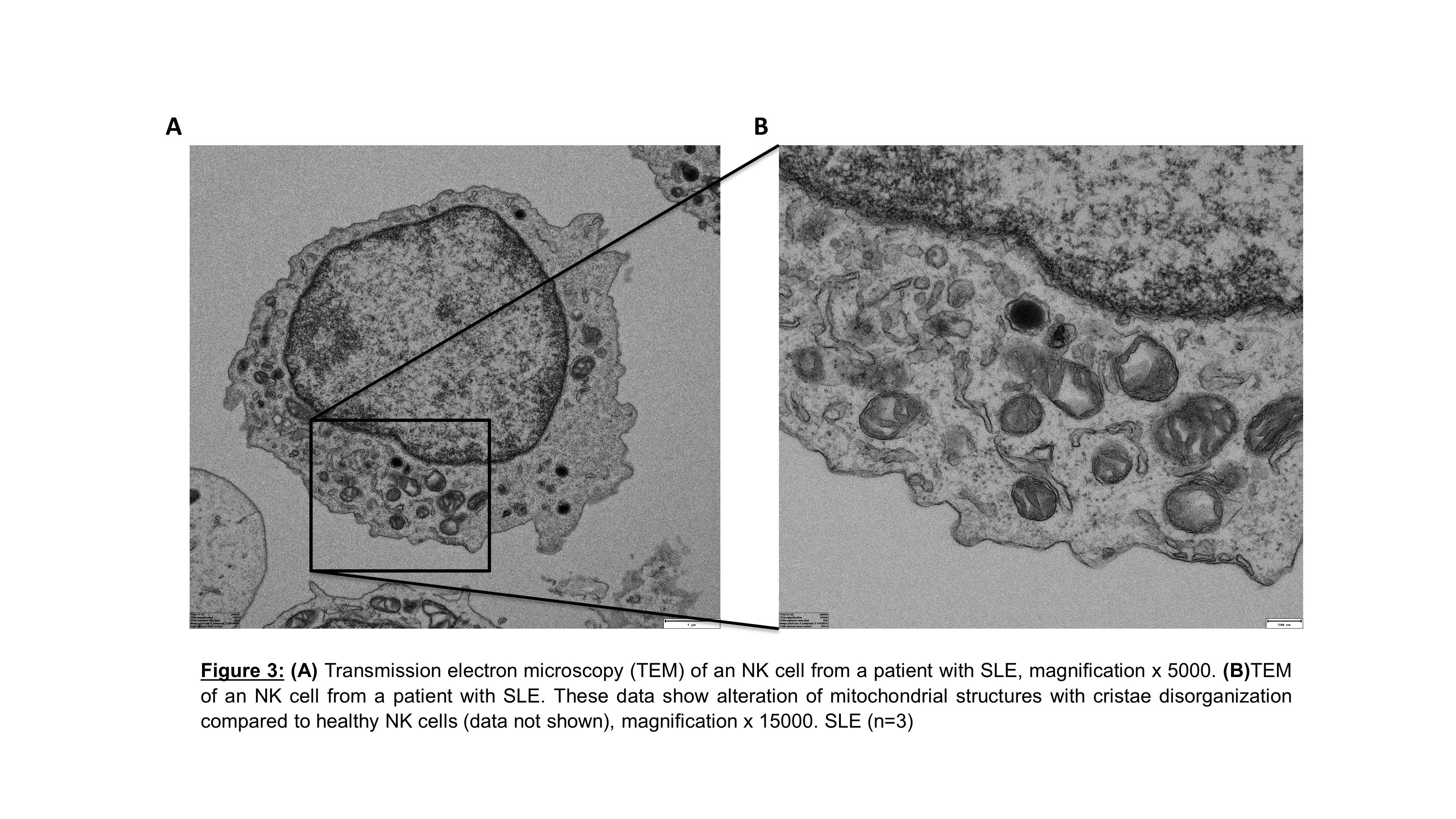
Results: We first examined the cellular metabolism of SLE NK cells in comparison to healthy NK cells. We observed that OXPHOS is significantly increased in SLE NK cells, while their glycolysis is similar to that of healthy controls. To further understand these results, we show that mitochondrial mass is increased, while mitochondrial activity (mitochondrial membrane potential/mass ratio) is decreased in SLE NK cells, suggesting an accumulation of dysfunctional mitochondria. Mitochondria from SLE NK cells displayed increased superoxide levels and elevated lysosomal acidification, compared to healthy NK cells. Analysis of SLE NK cells by confocal microscopy together with qPCR analysis, consistently confirmed the increased mitochondrial mass, in SLE NK cells. Moreover, transmission electron microscopy examination of mitochondria in SLE NK cells showed cristae disorganization.
Conclusion: SLE NK cells exhibit impaired mitochondrial respiration, which is linked to an accumulation of mitochondria with cristae disorganization. These results suggest an alteration in mitochondrial degradation in SLE NK cells related to alterations in mitophagy process. Taken together, these data suggest that impaired mitochondrial function and turnover represent a major feature of SLE pathogenesis.
Disclosures: N. Fluder, None; M. Humbel, None; c. Ribi, None; D. Comte, None.
Background/Purpose: Systemic lupus erythematosus (SLE) is a systemic inflammatory disorder, which involves a loss of tolerance and development of autoantibodies. The role of Natural Killer (NK) cells in SLE remains poorly understood. Studies have shown that SLE NK cells are decreased in the peripheral blood, exhibit reduced cytotoxicity, and impaired cytokine production. To date, few studies have explored the molecular mechanisms underlying NK cell dysfunction in SLE.
Herein, we examined metabolic alterations of human SLE NK cells, compared to healthy controls. First, we characterized the glycolysis and oxidative phosphorylation (OXPHOS). Then, we examined the mitochondrial fitness of SLE NK cells.
Methods: SLE and healthy NK cells were isolated from peripheral blood mononuclear cells (PBMC). Glycolysis and OXPHOS were assessed by measuring the extracellular acidification rate (ECAR) and the oxidative consumption rate (OCR), respectively, using the XFe96 Seahorse. Mitochondrial function (membrane potential, mitochondrial mass), mitochondrial superoxide levels and acidification of lysosomes were assessed by flow cytometry. In parallel, mitochondrial membrane potential and mass were observed and quantified, by confocal microscopy. Further, mitochondrial ultrastructure was investigated by transmission electron microscopy. Finally, the ratio of mitochondrial DNA over nuclear DNA was measured using quantitative polymerase chain reaction (qPCR).
Results: We first examined the cellular metabolism of SLE NK cells in comparison to healthy NK cells. We observed that OXPHOS is significantly increased in SLE NK cells, while their glycolysis is similar to that of healthy controls. To further understand these results, we show that mitochondrial mass is increased, while mitochondrial activity (mitochondrial membrane potential/mass ratio) is decreased in SLE NK cells, suggesting an accumulation of dysfunctional mitochondria. Mitochondria from SLE NK cells displayed increased superoxide levels and elevated lysosomal acidification, compared to healthy NK cells. Analysis of SLE NK cells by confocal microscopy together with qPCR analysis, consistently confirmed the increased mitochondrial mass, in SLE NK cells. Moreover, transmission electron microscopy examination of mitochondria in SLE NK cells showed cristae disorganization.
Conclusion: SLE NK cells exhibit impaired mitochondrial respiration, which is linked to an accumulation of mitochondria with cristae disorganization. These results suggest an alteration in mitochondrial degradation in SLE NK cells related to alterations in mitophagy process. Taken together, these data suggest that impaired mitochondrial function and turnover represent a major feature of SLE pathogenesis.



Disclosures: N. Fluder, None; M. Humbel, None; c. Ribi, None; D. Comte, None.

