Back
Poster Session B
Reproductive health
Session: (0939–0969) Reproductive Issues in Rheumatic Disorders Poster
0955: Unfavorable Pregnancy Outcome Is Significantly Associated with Corticosteroid Exposure During Pregnancy in Women with RA: Analysis of the Prospective GR2 Cohort
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- SH
SABRINA HAMROUN, MMed
Rheumatology Department, University Hospital of Cochin, Paris
Paris, France
Abstract Poster Presenter(s)
SABRINA HAMROUN1, Marion Couderc2, Rene-Marc Flipo3, Laure Gossec4, Christophe Richez5, Rakiba Belkhir6, Aline Frazier-Mironer7, Valerie Devauchelle8, Hubert Marotte9, Jérémie SELLAM10, elisabeth gervais11, Alban DEROUX12, Cédric Lukas13, Emmanuelle Dernis14, Emmanuel Chatelus15 and Anna Molto16, 1Rheumatology Department, University Hospital of Cochin, Paris, Paris, France, 2University Hospital, Clermont-Ferrand, France, 3CHU Lille, Boulogne-Billancourt, France, 4Sorbonne Université, Paris, France, 5Université de Bordeaux, Bordeaux, France, 6Rheumatology departement, Bicêtre, Paris-Saclay university, Le Kremlin Bicêtre, France, 7APHP Hôpital Lariboisire, Paris, France, 8Université de Bretagne Occidentale, Brest, France, 9INSERM 1059, Saint-Etienne, France, 10Sorbonne Universite, AP-HP, Saint-Antoine hospital, Paris, France, 11chu poitiers, Poitiers, France, 12CHU Grenoble, Grenoble, France, 13University Hospital Centre Montpellier, University of Montpellier, Montpellier, France, 14LE MANS general hospital, LE MANS, France, 15University Hospital of Strasbourg, Strasbourg, France, 16Rheumatology Department, Cochin Hospital, APHP, Paris, France
Background/Purpose: Rheumatoid arthritis (RA) is one of the most common chronic inflammatory diseases and regularly affects women of childbearing age. However, there is limited knowledge about the impact of the disease and its treatment on pregnancy. The aim of the study was to determine the factors associated with adverse pregnancy outcome in women with RA.
Methods: All RA patients (diagnosis according to the Rheumatologist) included in the national multicenter GR2 cohort from 2015 to June 2021 were included in the analysis. Patients could be included either with a pregnancy wish (i.e., preconceptional period) or because of a clinical pregnancy (< 12 weeks of gestation). The main endpoint was favorable pregnancy outcome, a composite outcome defined as a live birth at term ≥ 37 gestation weeks of a healthy newborn with a weight greater than the 10th percentile. Disease activity was defined by a DAS28-CRP score > 3.2 at least once during pregnancy. We performed a multilevel logistic regression model, in which we considered patient and center random effects (patient random effect for some women included in the cohort two times). We used a multiple imputation procedure to address missing data among the explanatory variables. Results are presented as an odds ratio (OR) with confidence interval (CI).
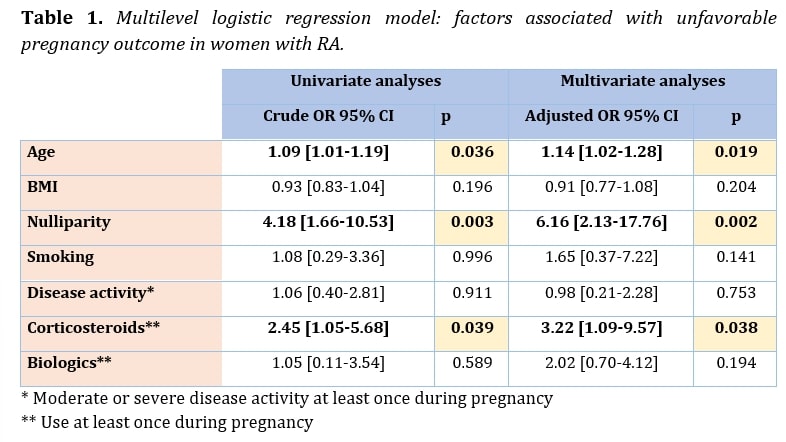
Results: Among the 167 pregnancies in women with RA included in the GR2 cohort, 92 were retained for analysis of obstetrical outcome. Of these, 43 (46.2%), 8 (7.9%), 40 (43.5%) were exposed to corticosteroid, NSAID and biologics at least once during pregnancy, respectively. A moderate or severe disease activity at least once during pregnancy was found in 20 (21.8%) pregnancies. A live birth was found in 83 (90.2%) women, including 69 (83.1%) full-term births. Early miscarriages were observed in 9 (0.1%) women. A caesarean section was performed in 22 (23.9%) cases. A favorable pregnancy outcome was found in 52 (56.5%) of the women. Unfavorable pregnancy outcome was mainly due to prematurity and small for gestational age, observed in 14 (16.9%) and 17 (20.5%), respectively. The multivariate model adjusted for age, BMI, nulliparity, active disease during pregnancy, smoking, and exposure to biologics and corticosteroids during pregnancy found an association between an unfavorable pregnancy outcome and nulliparity (OR 6.2 95% CI [2.1-17.8] p = 0.002), age (OR (per year) 1.1 95% CI [1.0-1.3] p = 0.02) and exposition to corticosteroids during pregnancy (OR 3.2 95% CI [1.1-9.6] p = 0.04).
Conclusion: This study provides original results on pregnancy in women with RA. It found a favorable pregnancy outcome in 56.5% of women. Unfavorable pregnancy outcome was associated with age, nulliparity and corticosteroids use during pregnancy, which argues for their careful use during pregnancy.
Disclosures: S. HAMROUN, None; M. Couderc, None; R. Flipo, Merck/MSD, Novartis, Janssen; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; C. Richez, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Galapados, GlaxoSmithKlein(GSK), Eli Lilly, Novartis, Pfizer; R. Belkhir, None; A. Frazier-Mironer, None; V. Devauchelle, Pfizer, Novartis, AbbVie/Abbott, Novartis, Bristol-Myers Squibb(BMS), Roche-Chugai, Galapados; H. Marotte, AbbVie/Abbott, Amgen, Biogen, Bristol-Myers Squibb(BMS), CellTrion HealthCare, Fresenius Kabi, HealthCare, Janssen, Eli Lilly, Nordic Pharma, Novartis, Medac, Pfizer, Merck/MSD, Galapagos, UCB; J. SELLAM, None; e. gervais, None; A. DEROUX, None; C. Lukas, None; E. Dernis, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Nordic Pharma France, Novartis, UCB; E. Chatelus, None; A. Molto, None.
Background/Purpose: Rheumatoid arthritis (RA) is one of the most common chronic inflammatory diseases and regularly affects women of childbearing age. However, there is limited knowledge about the impact of the disease and its treatment on pregnancy. The aim of the study was to determine the factors associated with adverse pregnancy outcome in women with RA.
Methods: All RA patients (diagnosis according to the Rheumatologist) included in the national multicenter GR2 cohort from 2015 to June 2021 were included in the analysis. Patients could be included either with a pregnancy wish (i.e., preconceptional period) or because of a clinical pregnancy (< 12 weeks of gestation). The main endpoint was favorable pregnancy outcome, a composite outcome defined as a live birth at term ≥ 37 gestation weeks of a healthy newborn with a weight greater than the 10th percentile. Disease activity was defined by a DAS28-CRP score > 3.2 at least once during pregnancy. We performed a multilevel logistic regression model, in which we considered patient and center random effects (patient random effect for some women included in the cohort two times). We used a multiple imputation procedure to address missing data among the explanatory variables. Results are presented as an odds ratio (OR) with confidence interval (CI).
Results: Among the 167 pregnancies in women with RA included in the GR2 cohort, 92 were retained for analysis of obstetrical outcome. Of these, 43 (46.2%), 8 (7.9%), 40 (43.5%) were exposed to corticosteroid, NSAID and biologics at least once during pregnancy, respectively. A moderate or severe disease activity at least once during pregnancy was found in 20 (21.8%) pregnancies. A live birth was found in 83 (90.2%) women, including 69 (83.1%) full-term births. Early miscarriages were observed in 9 (0.1%) women. A caesarean section was performed in 22 (23.9%) cases. A favorable pregnancy outcome was found in 52 (56.5%) of the women. Unfavorable pregnancy outcome was mainly due to prematurity and small for gestational age, observed in 14 (16.9%) and 17 (20.5%), respectively. The multivariate model adjusted for age, BMI, nulliparity, active disease during pregnancy, smoking, and exposure to biologics and corticosteroids during pregnancy found an association between an unfavorable pregnancy outcome and nulliparity (OR 6.2 95% CI [2.1-17.8] p = 0.002), age (OR (per year) 1.1 95% CI [1.0-1.3] p = 0.02) and exposition to corticosteroids during pregnancy (OR 3.2 95% CI [1.1-9.6] p = 0.04).
Conclusion: This study provides original results on pregnancy in women with RA. It found a favorable pregnancy outcome in 56.5% of women. Unfavorable pregnancy outcome was associated with age, nulliparity and corticosteroids use during pregnancy, which argues for their careful use during pregnancy.

Disclosures: S. HAMROUN, None; M. Couderc, None; R. Flipo, Merck/MSD, Novartis, Janssen; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; C. Richez, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Galapados, GlaxoSmithKlein(GSK), Eli Lilly, Novartis, Pfizer; R. Belkhir, None; A. Frazier-Mironer, None; V. Devauchelle, Pfizer, Novartis, AbbVie/Abbott, Novartis, Bristol-Myers Squibb(BMS), Roche-Chugai, Galapados; H. Marotte, AbbVie/Abbott, Amgen, Biogen, Bristol-Myers Squibb(BMS), CellTrion HealthCare, Fresenius Kabi, HealthCare, Janssen, Eli Lilly, Nordic Pharma, Novartis, Medac, Pfizer, Merck/MSD, Galapagos, UCB; J. SELLAM, None; e. gervais, None; A. DEROUX, None; C. Lukas, None; E. Dernis, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Nordic Pharma France, Novartis, UCB; E. Chatelus, None; A. Molto, None.

