Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0913–0938) RA – Treatment Poster II
0928: Use of Disease Modifying Anti-rheumatic Drugs and Risk of Multiple Myeloma in US Veterans with Rheumatoid Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- NS
Namrata Singh, MD, MSc
University of Washington
Bellevue, WA, United States
Abstract Poster Presenter(s)
Namrata Singh1, Alexander Peterson2, Aaron Baraff2, Sarah Chung3, David Coffey4, Bryant England5, Pankti Reid6, Joshua Baker7, Jennifer Barton8, Nicholas Smith9, Ted Mikuls10 and Noel Weiss3, 1University of Washington, Bellevue, WA, 2VA Puget Sound, SEATTLE, WA, 3University of Washington, Seattle, WA, 4University of Miami, Miami, FL, 5University of Nebraska Medical Center, Omaha, NE, 6University of Chicago Medical Center, Chicago, IL, 7University of Pennsylvania, Philadelphia, PA, 8VA Portland Health Care System/OHSU, Portland, OR, 9VA Puget Sound/University of Washington, Seattle, WA, 10Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Biologic (b) and targeted synthetic (ts) DMARDs used in the management of rheumatoid arthritis (RA) target inflammatory pathways implicated in the pathogenesis of multiple myeloma (MM). Yet little is known about the association between use of bDMARDs or tsDMARDs in RA and the incidence of MM. Our objective was to estimate the associations between b/tsDMARD use and the risk of MM among persons with RA using Veterans Health Administration (VHA) data.
Methods: In this retrospective cohort study, we identified patients >18 years of age diagnosed with RA in any United States VHA facility from 1/1/2002 and 12/31/2018. Diagnosis of RA was defined with use of two or more International Classification of Diseases Version 9 or 10 (ICD9 or ICD10) codes for RA at least 7 days apart but no more than 365 days apart and a prescription for a csDMARD within 90 days of the first RA diagnosis (index date). Medication data was derived from the outpatient prescription fills, bar coded medication administration (BCMA), and intravenous (IV) data domains. The csDMARDs included in these analyses were: methotrexate, sulfasalazine, leflunomide, and hydroxychloroquine. The bDMARDs included were tumor necrosis factor inhibitors (TNFi) and non-TNFi biologics such as tocilizumab, rituximab, abatacept, and biosimilars; tsDMARD was tofacitinib. Patients with MM before the diagnosis of RA were excluded. Incident MM was determined by 1 or more ICD9/10 code or ICD-oncology codes. Multivariable Cox proportional hazards model with time-varying exposure was performed to estimate the hazard ratio for developing MM among those during and following the use of a b/tsDMARD relative to b/tsDMARD-naïve persons adjusting for age, gender, race, and ethnicity.
Results: 27,540 veterans with RA met study eligibility criteria, of whom 8,322 (30%) had ever taken a b/tsDMARD during follow up. Over the study period there were 77 incident MM over a total of 192,000 person years (median follow-up 5.8 years). There were 55 events in users of csDMARDs, an incidence rate (IR) of 0.40 (95% CI 0.30-0.52) per 1000 person-years and 22 in persons currently or formerly using b/tsDMARDs (IR 0.41, 0.25-0.61 per 1000 person years). The unadjusted hazard ratio for MM following b/tsDMARD use relative to csDMARD only use was 1.04 (0.63, 1.73), which increased to 1.28 (0.76, 2.16) after adjusting for demographic characteristics (Table 1).
Conclusion: In this nationwide VA study, rates of MM were not different among b/tsDMARD and csDMARD users after accounting for demographics. Of note, the median interval from initiation of a bDMARD to the end of follow-up was approximately 5.8 years, which does not allow for an examination of a possible longer-term influence.
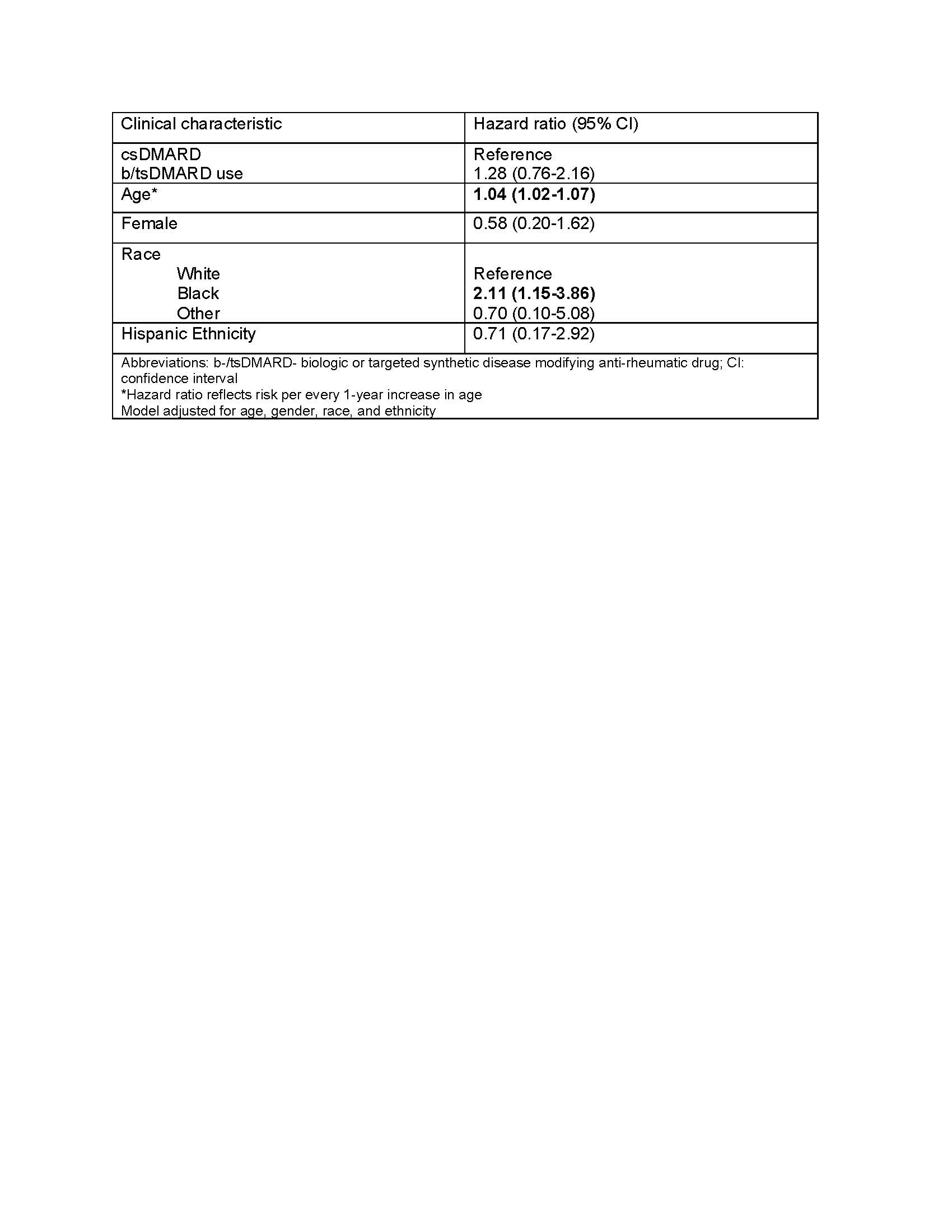 Table 1. Multivariable Cox proportional hazards model for association between use of disease modifying anti-rheumatic drugs and incident multiple myeloma.
Table 1. Multivariable Cox proportional hazards model for association between use of disease modifying anti-rheumatic drugs and incident multiple myeloma.
Disclosures: N. Singh, None; A. Peterson, None; A. Baraff, None; S. Chung, None; D. Coffey, None; B. England, Boehringer-Ingelheim; P. Reid, provisional patent; J. Baker, Bristol-Myers Squibb(BMS), RediTrex, Pfizer; J. Barton, None; N. Smith, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; N. Weiss, None.
Background/Purpose: Biologic (b) and targeted synthetic (ts) DMARDs used in the management of rheumatoid arthritis (RA) target inflammatory pathways implicated in the pathogenesis of multiple myeloma (MM). Yet little is known about the association between use of bDMARDs or tsDMARDs in RA and the incidence of MM. Our objective was to estimate the associations between b/tsDMARD use and the risk of MM among persons with RA using Veterans Health Administration (VHA) data.
Methods: In this retrospective cohort study, we identified patients >18 years of age diagnosed with RA in any United States VHA facility from 1/1/2002 and 12/31/2018. Diagnosis of RA was defined with use of two or more International Classification of Diseases Version 9 or 10 (ICD9 or ICD10) codes for RA at least 7 days apart but no more than 365 days apart and a prescription for a csDMARD within 90 days of the first RA diagnosis (index date). Medication data was derived from the outpatient prescription fills, bar coded medication administration (BCMA), and intravenous (IV) data domains. The csDMARDs included in these analyses were: methotrexate, sulfasalazine, leflunomide, and hydroxychloroquine. The bDMARDs included were tumor necrosis factor inhibitors (TNFi) and non-TNFi biologics such as tocilizumab, rituximab, abatacept, and biosimilars; tsDMARD was tofacitinib. Patients with MM before the diagnosis of RA were excluded. Incident MM was determined by 1 or more ICD9/10 code or ICD-oncology codes. Multivariable Cox proportional hazards model with time-varying exposure was performed to estimate the hazard ratio for developing MM among those during and following the use of a b/tsDMARD relative to b/tsDMARD-naïve persons adjusting for age, gender, race, and ethnicity.
Results: 27,540 veterans with RA met study eligibility criteria, of whom 8,322 (30%) had ever taken a b/tsDMARD during follow up. Over the study period there were 77 incident MM over a total of 192,000 person years (median follow-up 5.8 years). There were 55 events in users of csDMARDs, an incidence rate (IR) of 0.40 (95% CI 0.30-0.52) per 1000 person-years and 22 in persons currently or formerly using b/tsDMARDs (IR 0.41, 0.25-0.61 per 1000 person years). The unadjusted hazard ratio for MM following b/tsDMARD use relative to csDMARD only use was 1.04 (0.63, 1.73), which increased to 1.28 (0.76, 2.16) after adjusting for demographic characteristics (Table 1).
Conclusion: In this nationwide VA study, rates of MM were not different among b/tsDMARD and csDMARD users after accounting for demographics. Of note, the median interval from initiation of a bDMARD to the end of follow-up was approximately 5.8 years, which does not allow for an examination of a possible longer-term influence.
 Table 1. Multivariable Cox proportional hazards model for association between use of disease modifying anti-rheumatic drugs and incident multiple myeloma.
Table 1. Multivariable Cox proportional hazards model for association between use of disease modifying anti-rheumatic drugs and incident multiple myeloma.Disclosures: N. Singh, None; A. Peterson, None; A. Baraff, None; S. Chung, None; D. Coffey, None; B. England, Boehringer-Ingelheim; P. Reid, provisional patent; J. Baker, Bristol-Myers Squibb(BMS), RediTrex, Pfizer; J. Barton, None; N. Smith, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; N. Weiss, None.

