Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0974–1003) SLE – Treatment Poster II
0997: Withdrawal of Maintenance Glucocorticoid versus Other Immunosuppressants Among Patients with Systemic Lupus Erythematosus in Long Term Clinical Remission: Interim Analysis of a Non-inferiority Randomised Controlled Trial
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- AG
Aishwarya Gopal, MD, MBBS
Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER)
Pondicherry, Puducherry, India
Abstract Poster Presenter(s)
Aishwarya Gopal1, Chengappa Kavadichanda2, Devender Bairwa3, Molly Mary Thabah4 and Vir Singh Negi5, 1Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, Puducherry, India, 2JIPMER, Pondicherry, Puducherry, India, 3Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, Puducherry, India, 4JIPMER, Puducherry, Puducherry, India, 5AIIMS Bilaspur, Puducherry, Puducherry, India
Background/Purpose: Attempts to stop glucocorticoids among Systemic Lupus Erythematosus(SLE) patients in long term remission have been successful. Continuing other immunosuppressive (IS) agents indefinitely is currently the norm and this approach results in significant infectious, psychological and economic burden on patients. Moreover, there is no pathophysiological basis to withdraw glucocorticoids over the other IS agents, except for the indirect evidence of higher adverse effects with continued low dose steroid use. Hence, there is a need to assess, if the approach to taper steroids first is the right way in lupus. The objective of this study is to compare the outcome of glucocorticoid withdrawal versus immunosuppressant withdrawal in patients of SLE in clinical remission
Methods: This is a single centre, non-inferiority, open-label stratified randomized (1:1) controlled trial. Patients with SLE who were on IS for at least 3 years, were in remission (DORIS definition) for a minimum of one-year preceding screening for the study and were on stable prednisolone dose of ≤7.5mg/day plus a maintenance non-biological IS were included. Stratification was done based on the duration of IS use (< 3 years and ≥ 3 years), and the presence of proliferative lupus nephritis. All the patients continued HCQ during the study. Prednisolone or IS was tapered gradually over 3 months after randomisation. The primary endpoint was the proportion experiencing a flare [SELENA-SLEDAI flare index (SFI)] and the British Isles Lupus Assessment Group (BILAG) index at 52 weeks. Secondary endpoints were time to flare and severity of flare (SFI and BILAG). Non-inferiority margin considered was 10%. For this abstract, data of the maximum available follow up period for each patient was used.
Results: A total of 117 patients were randomised into steroid withdrawal (n=58) and IS withdrawal (n=59). Baseline characteristics of the two groups at randomization are summarized in Table 1. Overall, 72% of the patients were in prolonged remission (DORIS remission on treatment >5 years). The mean follow up duration was 39 ± 12 weeks.
There was a total of 22 flares of which 19 were mild-moderate and 3 were severe flares (SFI). Proportion of patients experiencing a flare was numerically higher in the steroid withdrawal group (15 vs 7 patients, RR 2.1; 95%CI (0.9-4.8), p=0.06). (Table 2). Time to first flare was 27 days and 18 days following intervention in steroid and IS group respectively. Severity of flare and time of first flare were not significantly different between the two groups (HR=0.42, 95%CI (0.17-1.03), p=0.051) (figure 1). Severe flares were 2(3.4%) vs 1(1.7%) and mild-moderate flares were 13(22.4%) and 6(10.2%) in the steroid versus IS withdrawal arm respectively. Musculoskeletal (n=10) and mucocutaneous (n=9) were the commonest flares. There was one death due to MI secondary to ASCVD in a patient on IS withdrawal.
Conclusion: Immunosuppressant withdrawal is non-inferior to steroid withdrawal in patients with SLE in long term clinical remission. Identifying the individuals who are likely to flare is of utmost importance which can pave the way for a more personalised drug withdrawal approach.
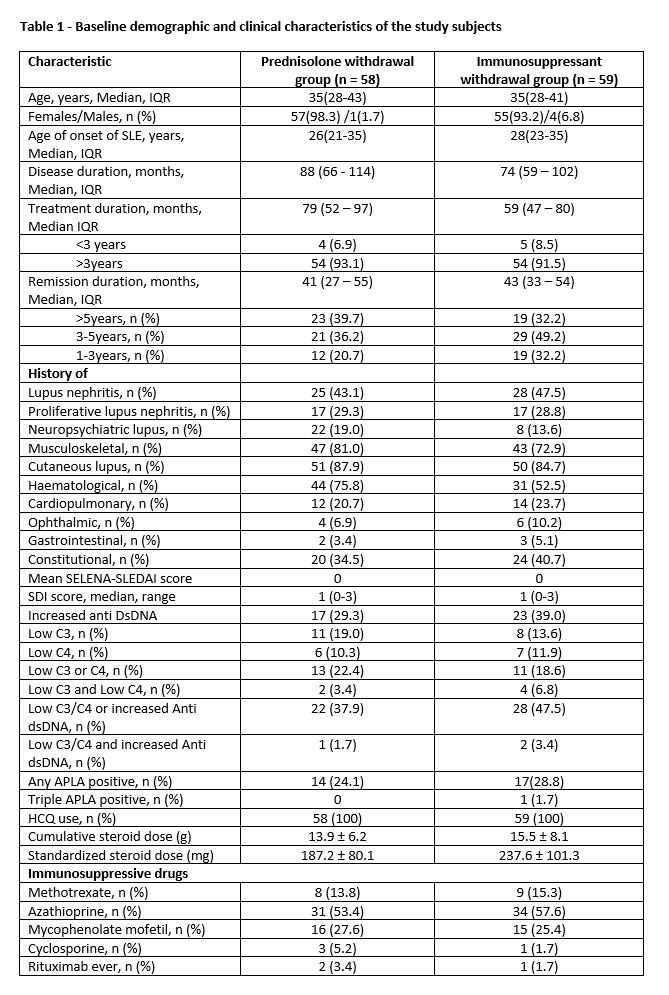 Table 1 - Baseline demographic and clinical characteristics of the study subjects
Table 1 - Baseline demographic and clinical characteristics of the study subjects
.jpg) Table 2 - Results of primary and secondary endpoints at maximum available follow up period (mean follow-up duration: 39 ± 12 weeks)
Table 2 - Results of primary and secondary endpoints at maximum available follow up period (mean follow-up duration: 39 ± 12 weeks)
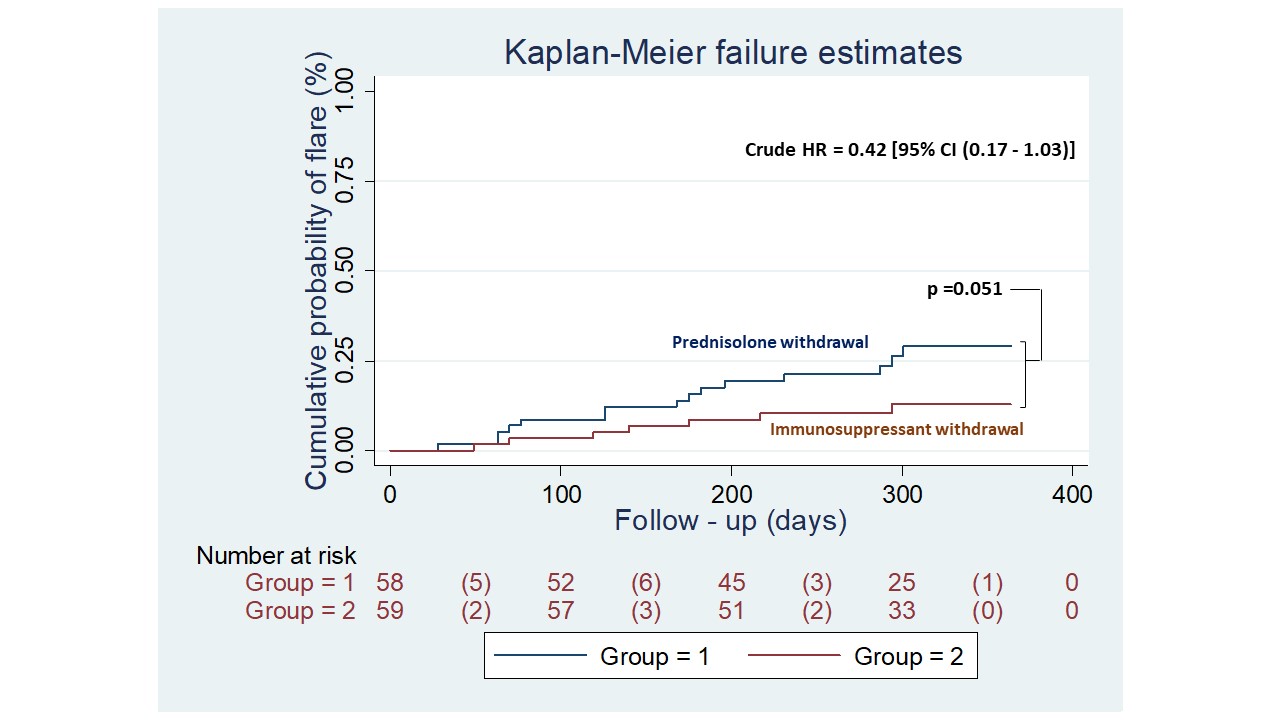 Figure 1 Kaplan-Meier estimates of the cumulative probability of flare for patients in the prednisone withdrawal and immunosuppressant withdrawal groups. Clinically quiescent SLE patients were allocated at day 0 to stop prednisolone (blue line) or immunosuppressant (red line) and were followed for 52 weeks. Each corner represents either a censored patient or a lupus flare. The number in parentheses represents a lupus flare, defined by the SELENA-SLEDAI flare index. Patients who had a flare in any organ system were recorded. Kaplan-Meier plots show the percentage of patients who flared in any organ system. Curves were compared using the log-rank test. Crude HR was calculated using a proportional risk COX model. SLE, systemic lupus erythematosus.
Figure 1 Kaplan-Meier estimates of the cumulative probability of flare for patients in the prednisone withdrawal and immunosuppressant withdrawal groups. Clinically quiescent SLE patients were allocated at day 0 to stop prednisolone (blue line) or immunosuppressant (red line) and were followed for 52 weeks. Each corner represents either a censored patient or a lupus flare. The number in parentheses represents a lupus flare, defined by the SELENA-SLEDAI flare index. Patients who had a flare in any organ system were recorded. Kaplan-Meier plots show the percentage of patients who flared in any organ system. Curves were compared using the log-rank test. Crude HR was calculated using a proportional risk COX model. SLE, systemic lupus erythematosus.
Disclosures: A. Gopal, None; C. Kavadichanda, None; D. Bairwa, None; M. Thabah, None; V. Negi, None.
Background/Purpose: Attempts to stop glucocorticoids among Systemic Lupus Erythematosus(SLE) patients in long term remission have been successful. Continuing other immunosuppressive (IS) agents indefinitely is currently the norm and this approach results in significant infectious, psychological and economic burden on patients. Moreover, there is no pathophysiological basis to withdraw glucocorticoids over the other IS agents, except for the indirect evidence of higher adverse effects with continued low dose steroid use. Hence, there is a need to assess, if the approach to taper steroids first is the right way in lupus. The objective of this study is to compare the outcome of glucocorticoid withdrawal versus immunosuppressant withdrawal in patients of SLE in clinical remission
Methods: This is a single centre, non-inferiority, open-label stratified randomized (1:1) controlled trial. Patients with SLE who were on IS for at least 3 years, were in remission (DORIS definition) for a minimum of one-year preceding screening for the study and were on stable prednisolone dose of ≤7.5mg/day plus a maintenance non-biological IS were included. Stratification was done based on the duration of IS use (< 3 years and ≥ 3 years), and the presence of proliferative lupus nephritis. All the patients continued HCQ during the study. Prednisolone or IS was tapered gradually over 3 months after randomisation. The primary endpoint was the proportion experiencing a flare [SELENA-SLEDAI flare index (SFI)] and the British Isles Lupus Assessment Group (BILAG) index at 52 weeks. Secondary endpoints were time to flare and severity of flare (SFI and BILAG). Non-inferiority margin considered was 10%. For this abstract, data of the maximum available follow up period for each patient was used.
Results: A total of 117 patients were randomised into steroid withdrawal (n=58) and IS withdrawal (n=59). Baseline characteristics of the two groups at randomization are summarized in Table 1. Overall, 72% of the patients were in prolonged remission (DORIS remission on treatment >5 years). The mean follow up duration was 39 ± 12 weeks.
There was a total of 22 flares of which 19 were mild-moderate and 3 were severe flares (SFI). Proportion of patients experiencing a flare was numerically higher in the steroid withdrawal group (15 vs 7 patients, RR 2.1; 95%CI (0.9-4.8), p=0.06). (Table 2). Time to first flare was 27 days and 18 days following intervention in steroid and IS group respectively. Severity of flare and time of first flare were not significantly different between the two groups (HR=0.42, 95%CI (0.17-1.03), p=0.051) (figure 1). Severe flares were 2(3.4%) vs 1(1.7%) and mild-moderate flares were 13(22.4%) and 6(10.2%) in the steroid versus IS withdrawal arm respectively. Musculoskeletal (n=10) and mucocutaneous (n=9) were the commonest flares. There was one death due to MI secondary to ASCVD in a patient on IS withdrawal.
Conclusion: Immunosuppressant withdrawal is non-inferior to steroid withdrawal in patients with SLE in long term clinical remission. Identifying the individuals who are likely to flare is of utmost importance which can pave the way for a more personalised drug withdrawal approach.
 Table 1 - Baseline demographic and clinical characteristics of the study subjects
Table 1 - Baseline demographic and clinical characteristics of the study subjects.jpg) Table 2 - Results of primary and secondary endpoints at maximum available follow up period (mean follow-up duration: 39 ± 12 weeks)
Table 2 - Results of primary and secondary endpoints at maximum available follow up period (mean follow-up duration: 39 ± 12 weeks) Figure 1 Kaplan-Meier estimates of the cumulative probability of flare for patients in the prednisone withdrawal and immunosuppressant withdrawal groups. Clinically quiescent SLE patients were allocated at day 0 to stop prednisolone (blue line) or immunosuppressant (red line) and were followed for 52 weeks. Each corner represents either a censored patient or a lupus flare. The number in parentheses represents a lupus flare, defined by the SELENA-SLEDAI flare index. Patients who had a flare in any organ system were recorded. Kaplan-Meier plots show the percentage of patients who flared in any organ system. Curves were compared using the log-rank test. Crude HR was calculated using a proportional risk COX model. SLE, systemic lupus erythematosus.
Figure 1 Kaplan-Meier estimates of the cumulative probability of flare for patients in the prednisone withdrawal and immunosuppressant withdrawal groups. Clinically quiescent SLE patients were allocated at day 0 to stop prednisolone (blue line) or immunosuppressant (red line) and were followed for 52 weeks. Each corner represents either a censored patient or a lupus flare. The number in parentheses represents a lupus flare, defined by the SELENA-SLEDAI flare index. Patients who had a flare in any organ system were recorded. Kaplan-Meier plots show the percentage of patients who flared in any organ system. Curves were compared using the log-rank test. Crude HR was calculated using a proportional risk COX model. SLE, systemic lupus erythematosus.Disclosures: A. Gopal, None; C. Kavadichanda, None; D. Bairwa, None; M. Thabah, None; V. Negi, None.

