Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0589–0628) RA – Etiology and Pathogenesis Poster
0608: Wnt Pathway Regulators R-spondin 3 and Dickkopf-related Protein 3 Demarcate a Transcriptional Gradient That Drives Synovial Fibroblast Inflammatory Pathology in Rheumatoid Arthritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Alisa Mueller, MD, PhD
Fellow
Brigham and Women's Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Alisa Mueller1, Angela Zou2, SABA NAYAR3, Emily Taylor3, Triin Major3, David H Gardner3, Gerald FM Watts1, Adam Croft3, Roche Fibroblast Network Consortium4, Andrew Filer3, Christopher Buckley5, Kevin Wei6, ilya Korsunsky1, Soumya Raychaudhuri1 and Michael Brenner7, 1Brigham and Women's Hospital, Boston, MA, 2Harvard Medical School, Boston, MA, 3University of Birmingham, Birmingham, United Kingdom, 4Roche, Basel, Switzerland, 5University of Oxford, Oxford, United Kingdom, 6Brigham and Women's Hospital and Harvard Medical School, Boston, MA, 7Brigham and Women's Hospital, Harvard Medical School, Boston, MA
Background/Purpose: Synovial fibroblasts are key players in rheumatoid arthritis (RA) where they secrete inflammatory cytokines and directly instigate cartilage and bone destruction. However, most treatments are directed at lymphocyte populations, while none directly target these fibroblasts. Here, we report that non-canonical Wnt signaling drives a strong inflammatory gene expression signature among synovial fibroblasts that is associated with R-spondin 3 (RSPO3) and Dickkopf-related protein 3 (DKK3) expression gradients and correlated with RA and other inflammatory diseases.
Methods: We used single-cell and bulk RNA sequencing data from the Accelerating Medicines Partnership RA/SLE Network and the Roche Fibroblast Network Consortium to assess Wnt pathway member expression and gradients. We developed Wnt activation signatures using multivariate regression analysis of RNA sequencing data from human RA synovial fibroblasts stimulated in vitro with a combination of Wnt ligands at various doses and timepoints. We performed immunofluorescence on human RA synovial samples derived from clinical biopsy.
Results: In OA and RA synovium, Wnt pathway members are primarily expressed by fibroblasts (Fig. 1A). We investigated the effects of Wnt activation through RNA sequencing of human RA synovial fibroblasts stimulated with Wnt ligands in vitro. We identified genes that show a dose-responsive association with Wnt stimulation (Fig. 1B) and, unexpectedly, we found a striking enrichment of inflammation pathways (Fig. 1C). Furthermore, we identified an axis defined by reciprocal gradients of expression of RSPO3 and DKK3 that is orthogonal to the Notch-mediated lining-sublining gradient (Fig. 1D). We utilized our in vitro Wnt stimulation results to generate a Wnt enrichment score and found that expression of the positive Wnt regulator RSPO3 is associated with enhanced non-canonical Wnt pathway scores as well as activation markers (Fig. 1E). Moreover, donor-specific enhancement of Wnt5a and RSPO3 signatures was associated with decreased enrichment of signatures for DKK3, a putative Wnt inhibitor (Fig. 1F). We sought to identify the clinical relevance of this transcriptional gradient and validated the divergent protein expression of RSPO3 and DKK3 in synovial fibroblasts using multispectral immunofluorescence of human RA synovial biopsies (Fig. 2A). In synovial fibroblast bulk RNA sequencing data, RSPO3 signatures are correlated with Wnt5a signatures and show enhancement in active RA compared to OA, while DKK3 signatures are decreased in RA (Fig. 2B and 2C). Furthermore, in fibroblasts from salivary gland, lung, and gut from patients with Sjogren's disease, interstitial lung disease (ILD), and inflammatory bowel disease (IBD) compared with controls, RSPO3 and DKK3 show distinct expression patterns (Fig. 2D) with an association of Wnt5a and RSPO3 signatures in inflamed gut tissue (Fig. 2E).
Conclusion: We demonstrate that a Wnt-associated transcriptional gradient defined by Wnt modulators RSPO3 and DKK3 represents a novel mechanism associated with RA fibroblast inflammatory pathology. This work builds on existing groundwork for a class of stromal-targeted therapeutics for RA and other inflammatory diseases.
.jpg) Figure 1: Wnt signaling induces the expression of an inflammatory fibroblast signature and its activation is positively associated with expression of Wnt activator RSPO3. A) Wnt receptors and modulators in synovium are mainly expressed on fibroblasts including RSPO3 and DKK3. (B) Linear regression analysis of synovial fibroblasts incubated with Wnt ligands across concentrations and time points identifies genes that show increased expression in a dose-dependent manner, and (C) gene enrichment analysis reveals enhancement of genes related to inflammatory cytokine activity. (D) Stromal-directed single-cell data set shows DKK3 and RSPO3 exhibit reciprocal patterns of expression delineating a Wnt transcriptional axis that is orthogonal to the lining-sublining gradient defined by Notch. (E) Expression of Wnt enhancer RSPO3 is associated with an augmented non-canonical Wnt signature and the expression of cytokine and activation related genes as evaluated by gene enrichment analyses. (F) Gene signatures in synovial fibroblasts from individual donors show Wnt5a and RSPO3 signatures are positively correlated while DKK3 signature is reciprocally expressed. There is an enrichment of RSPO3 and Wnt5a signatures in RA fibroblasts.
Figure 1: Wnt signaling induces the expression of an inflammatory fibroblast signature and its activation is positively associated with expression of Wnt activator RSPO3. A) Wnt receptors and modulators in synovium are mainly expressed on fibroblasts including RSPO3 and DKK3. (B) Linear regression analysis of synovial fibroblasts incubated with Wnt ligands across concentrations and time points identifies genes that show increased expression in a dose-dependent manner, and (C) gene enrichment analysis reveals enhancement of genes related to inflammatory cytokine activity. (D) Stromal-directed single-cell data set shows DKK3 and RSPO3 exhibit reciprocal patterns of expression delineating a Wnt transcriptional axis that is orthogonal to the lining-sublining gradient defined by Notch. (E) Expression of Wnt enhancer RSPO3 is associated with an augmented non-canonical Wnt signature and the expression of cytokine and activation related genes as evaluated by gene enrichment analyses. (F) Gene signatures in synovial fibroblasts from individual donors show Wnt5a and RSPO3 signatures are positively correlated while DKK3 signature is reciprocally expressed. There is an enrichment of RSPO3 and Wnt5a signatures in RA fibroblasts.
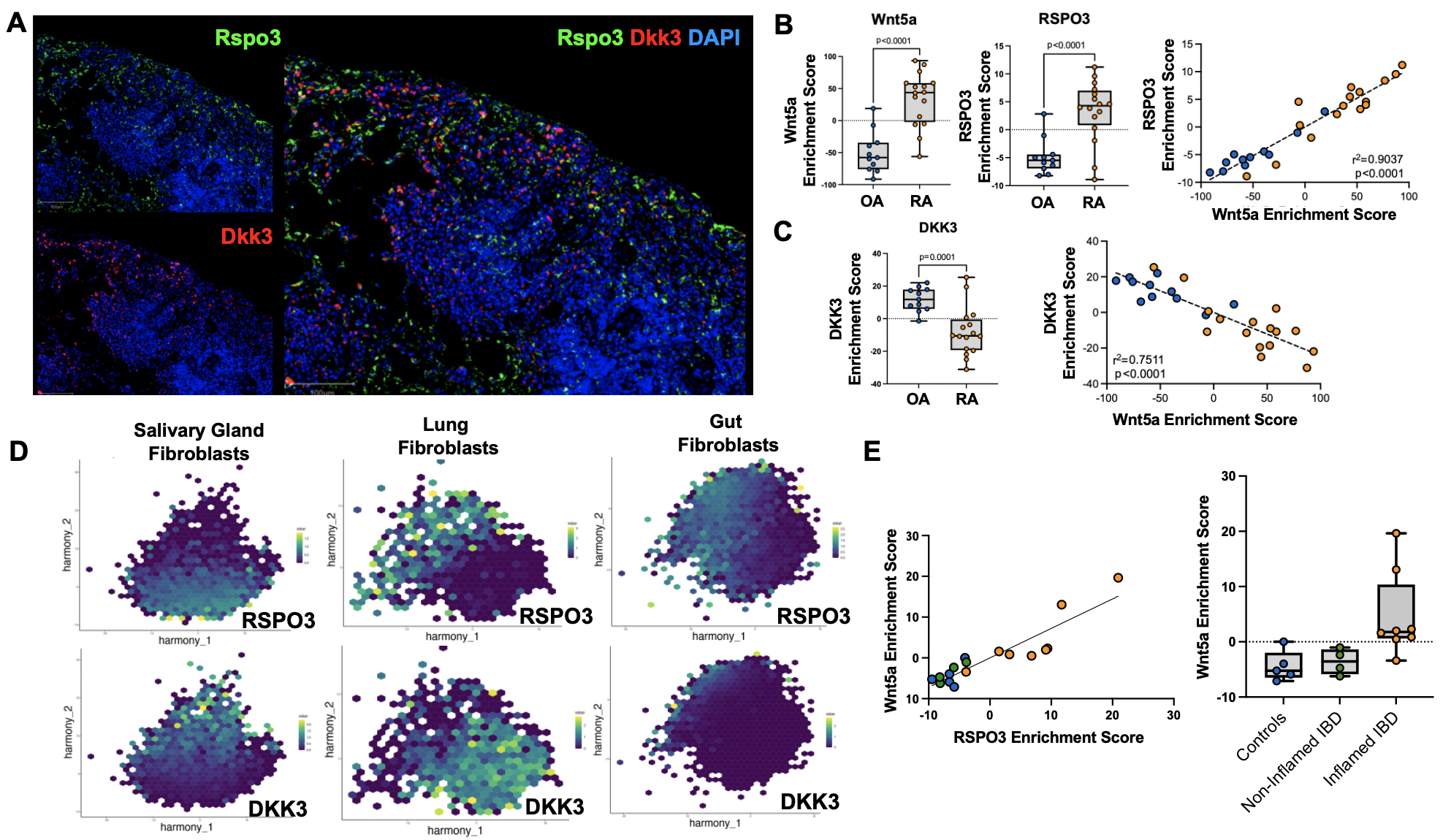 Figure 2: The Wnt-mediated transcriptional gradient in synovial fibroblasts demarcated by RSPO3 and DKK3 is associated with rheumatoid arthritis and other inflammatory diseases. A) Histologic analysis of Rspo3 and Dkk3 confirm protein expression of these markers on fibroblasts and a reciprocal pattern of expression. (B) Analysis of bulk RNA sequencing from AMP Phase 1 reveals that inflammatory RA synovial fibroblasts show higher Wnt5a and RSPO3 enrichment scores, which are positively correlated with one another. (C) DKK3 enrichment scores are conversely decreased in RA synovial fibroblasts, and the DKK3 signature is negatively correlated with the non-canonical Wnt signature. (D) Single-cell RNA sequencing analyses reveal that DKK3 and RSPO3 have distinct patterns of expression in salivary gland fibroblasts from patients with Sjogren’s disease and sicca symptoms, lung fibroblasts from patients with ILD and controls, and gut fibroblasts from patients with IBD patients and controls. (E) Pseudobulk analysis of gut fibroblasts from individual patients show a correlation of RSPO3 and Wnt5a enrichment scores, and Wnt5a scores are enhanced in fibroblasts from inflamed IBD gut tissue compared with non-inflamed IBD gut tissue and controls.
Figure 2: The Wnt-mediated transcriptional gradient in synovial fibroblasts demarcated by RSPO3 and DKK3 is associated with rheumatoid arthritis and other inflammatory diseases. A) Histologic analysis of Rspo3 and Dkk3 confirm protein expression of these markers on fibroblasts and a reciprocal pattern of expression. (B) Analysis of bulk RNA sequencing from AMP Phase 1 reveals that inflammatory RA synovial fibroblasts show higher Wnt5a and RSPO3 enrichment scores, which are positively correlated with one another. (C) DKK3 enrichment scores are conversely decreased in RA synovial fibroblasts, and the DKK3 signature is negatively correlated with the non-canonical Wnt signature. (D) Single-cell RNA sequencing analyses reveal that DKK3 and RSPO3 have distinct patterns of expression in salivary gland fibroblasts from patients with Sjogren’s disease and sicca symptoms, lung fibroblasts from patients with ILD and controls, and gut fibroblasts from patients with IBD patients and controls. (E) Pseudobulk analysis of gut fibroblasts from individual patients show a correlation of RSPO3 and Wnt5a enrichment scores, and Wnt5a scores are enhanced in fibroblasts from inflamed IBD gut tissue compared with non-inflamed IBD gut tissue and controls.
Disclosures: A. Mueller, None; A. Zou, None; S. NAYAR, None; E. Taylor, None; T. Major, None; D. Gardner, None; G. Watts, None; A. Croft, None; R. Fibroblast Network Consortium, Roche; A. Filer, None; C. Buckley, None; K. Wei, Gilead sciences, Mestag, nanoString, 10X Genomics; i. Korsunsky, Mestag Therapeutics Ltd.; S. Raychaudhuri, Mestag, Inc, Rheos Medicines, Janssen, Pfizer, Biogen; M. Brenner, GSK, 4FO Ventures, Mestag Therapeutics.
Background/Purpose: Synovial fibroblasts are key players in rheumatoid arthritis (RA) where they secrete inflammatory cytokines and directly instigate cartilage and bone destruction. However, most treatments are directed at lymphocyte populations, while none directly target these fibroblasts. Here, we report that non-canonical Wnt signaling drives a strong inflammatory gene expression signature among synovial fibroblasts that is associated with R-spondin 3 (RSPO3) and Dickkopf-related protein 3 (DKK3) expression gradients and correlated with RA and other inflammatory diseases.
Methods: We used single-cell and bulk RNA sequencing data from the Accelerating Medicines Partnership RA/SLE Network and the Roche Fibroblast Network Consortium to assess Wnt pathway member expression and gradients. We developed Wnt activation signatures using multivariate regression analysis of RNA sequencing data from human RA synovial fibroblasts stimulated in vitro with a combination of Wnt ligands at various doses and timepoints. We performed immunofluorescence on human RA synovial samples derived from clinical biopsy.
Results: In OA and RA synovium, Wnt pathway members are primarily expressed by fibroblasts (Fig. 1A). We investigated the effects of Wnt activation through RNA sequencing of human RA synovial fibroblasts stimulated with Wnt ligands in vitro. We identified genes that show a dose-responsive association with Wnt stimulation (Fig. 1B) and, unexpectedly, we found a striking enrichment of inflammation pathways (Fig. 1C). Furthermore, we identified an axis defined by reciprocal gradients of expression of RSPO3 and DKK3 that is orthogonal to the Notch-mediated lining-sublining gradient (Fig. 1D). We utilized our in vitro Wnt stimulation results to generate a Wnt enrichment score and found that expression of the positive Wnt regulator RSPO3 is associated with enhanced non-canonical Wnt pathway scores as well as activation markers (Fig. 1E). Moreover, donor-specific enhancement of Wnt5a and RSPO3 signatures was associated with decreased enrichment of signatures for DKK3, a putative Wnt inhibitor (Fig. 1F). We sought to identify the clinical relevance of this transcriptional gradient and validated the divergent protein expression of RSPO3 and DKK3 in synovial fibroblasts using multispectral immunofluorescence of human RA synovial biopsies (Fig. 2A). In synovial fibroblast bulk RNA sequencing data, RSPO3 signatures are correlated with Wnt5a signatures and show enhancement in active RA compared to OA, while DKK3 signatures are decreased in RA (Fig. 2B and 2C). Furthermore, in fibroblasts from salivary gland, lung, and gut from patients with Sjogren's disease, interstitial lung disease (ILD), and inflammatory bowel disease (IBD) compared with controls, RSPO3 and DKK3 show distinct expression patterns (Fig. 2D) with an association of Wnt5a and RSPO3 signatures in inflamed gut tissue (Fig. 2E).
Conclusion: We demonstrate that a Wnt-associated transcriptional gradient defined by Wnt modulators RSPO3 and DKK3 represents a novel mechanism associated with RA fibroblast inflammatory pathology. This work builds on existing groundwork for a class of stromal-targeted therapeutics for RA and other inflammatory diseases.
.jpg) Figure 1: Wnt signaling induces the expression of an inflammatory fibroblast signature and its activation is positively associated with expression of Wnt activator RSPO3. A) Wnt receptors and modulators in synovium are mainly expressed on fibroblasts including RSPO3 and DKK3. (B) Linear regression analysis of synovial fibroblasts incubated with Wnt ligands across concentrations and time points identifies genes that show increased expression in a dose-dependent manner, and (C) gene enrichment analysis reveals enhancement of genes related to inflammatory cytokine activity. (D) Stromal-directed single-cell data set shows DKK3 and RSPO3 exhibit reciprocal patterns of expression delineating a Wnt transcriptional axis that is orthogonal to the lining-sublining gradient defined by Notch. (E) Expression of Wnt enhancer RSPO3 is associated with an augmented non-canonical Wnt signature and the expression of cytokine and activation related genes as evaluated by gene enrichment analyses. (F) Gene signatures in synovial fibroblasts from individual donors show Wnt5a and RSPO3 signatures are positively correlated while DKK3 signature is reciprocally expressed. There is an enrichment of RSPO3 and Wnt5a signatures in RA fibroblasts.
Figure 1: Wnt signaling induces the expression of an inflammatory fibroblast signature and its activation is positively associated with expression of Wnt activator RSPO3. A) Wnt receptors and modulators in synovium are mainly expressed on fibroblasts including RSPO3 and DKK3. (B) Linear regression analysis of synovial fibroblasts incubated with Wnt ligands across concentrations and time points identifies genes that show increased expression in a dose-dependent manner, and (C) gene enrichment analysis reveals enhancement of genes related to inflammatory cytokine activity. (D) Stromal-directed single-cell data set shows DKK3 and RSPO3 exhibit reciprocal patterns of expression delineating a Wnt transcriptional axis that is orthogonal to the lining-sublining gradient defined by Notch. (E) Expression of Wnt enhancer RSPO3 is associated with an augmented non-canonical Wnt signature and the expression of cytokine and activation related genes as evaluated by gene enrichment analyses. (F) Gene signatures in synovial fibroblasts from individual donors show Wnt5a and RSPO3 signatures are positively correlated while DKK3 signature is reciprocally expressed. There is an enrichment of RSPO3 and Wnt5a signatures in RA fibroblasts.  Figure 2: The Wnt-mediated transcriptional gradient in synovial fibroblasts demarcated by RSPO3 and DKK3 is associated with rheumatoid arthritis and other inflammatory diseases. A) Histologic analysis of Rspo3 and Dkk3 confirm protein expression of these markers on fibroblasts and a reciprocal pattern of expression. (B) Analysis of bulk RNA sequencing from AMP Phase 1 reveals that inflammatory RA synovial fibroblasts show higher Wnt5a and RSPO3 enrichment scores, which are positively correlated with one another. (C) DKK3 enrichment scores are conversely decreased in RA synovial fibroblasts, and the DKK3 signature is negatively correlated with the non-canonical Wnt signature. (D) Single-cell RNA sequencing analyses reveal that DKK3 and RSPO3 have distinct patterns of expression in salivary gland fibroblasts from patients with Sjogren’s disease and sicca symptoms, lung fibroblasts from patients with ILD and controls, and gut fibroblasts from patients with IBD patients and controls. (E) Pseudobulk analysis of gut fibroblasts from individual patients show a correlation of RSPO3 and Wnt5a enrichment scores, and Wnt5a scores are enhanced in fibroblasts from inflamed IBD gut tissue compared with non-inflamed IBD gut tissue and controls.
Figure 2: The Wnt-mediated transcriptional gradient in synovial fibroblasts demarcated by RSPO3 and DKK3 is associated with rheumatoid arthritis and other inflammatory diseases. A) Histologic analysis of Rspo3 and Dkk3 confirm protein expression of these markers on fibroblasts and a reciprocal pattern of expression. (B) Analysis of bulk RNA sequencing from AMP Phase 1 reveals that inflammatory RA synovial fibroblasts show higher Wnt5a and RSPO3 enrichment scores, which are positively correlated with one another. (C) DKK3 enrichment scores are conversely decreased in RA synovial fibroblasts, and the DKK3 signature is negatively correlated with the non-canonical Wnt signature. (D) Single-cell RNA sequencing analyses reveal that DKK3 and RSPO3 have distinct patterns of expression in salivary gland fibroblasts from patients with Sjogren’s disease and sicca symptoms, lung fibroblasts from patients with ILD and controls, and gut fibroblasts from patients with IBD patients and controls. (E) Pseudobulk analysis of gut fibroblasts from individual patients show a correlation of RSPO3 and Wnt5a enrichment scores, and Wnt5a scores are enhanced in fibroblasts from inflamed IBD gut tissue compared with non-inflamed IBD gut tissue and controls. Disclosures: A. Mueller, None; A. Zou, None; S. NAYAR, None; E. Taylor, None; T. Major, None; D. Gardner, None; G. Watts, None; A. Croft, None; R. Fibroblast Network Consortium, Roche; A. Filer, None; C. Buckley, None; K. Wei, Gilead sciences, Mestag, nanoString, 10X Genomics; i. Korsunsky, Mestag Therapeutics Ltd.; S. Raychaudhuri, Mestag, Inc, Rheos Medicines, Janssen, Pfizer, Biogen; M. Brenner, GSK, 4FO Ventures, Mestag Therapeutics.

