Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1150–1165) Spondyloarthritis Including PsA – Basic Science Poster
1157: Bacterial Indole Associated with Spondyloarthritis-Related Dysbiosis Contributes to Enhanced Th17 Immunity
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- BS
Brenda Seymour, BS
University of Colorado
Denver, CO, United States
Abstract Poster Presenter(s)
Brenda Seymour1, Brandon Trent2, Brendan Allen3, Adam Berlinberg1 and Kristi Kuhn3, 1University of Colorado Anschutz Medical Campus, Denver, CO, 2University of Colorado Anschutz Medical Campus, Eugene, OR, 3University of Colorado Anschutz Medical Campus, Aurora, CO
Background/Purpose: While intestinal dysbiosis and the Th17 pathway are linked to the pathophysiology of spondyloarthritis (SpA), the mechanisms by which this occurs are unknown. We previously linked through microbiome metagenome and intestinal metabolome analyses the presence of indoles derived from bacterial metabolism of tryptophan (Trp) in patients with SpA. We then utilized the collagen-induced arthritis (CIA) model to investigate the role of indole in inflammatory arthritis because the Th17 pathway is required for CIA. We demonstrated that both the microbiome and dietary Trp are required for CIA, and that indole supplementation in either a Trp-deficient or microbiome-deficient setting fulfills this requirement. This led us to hypothesize that indole promotes inflammatory arthritis through enhanced Th17 immunity.
Methods: 6wk-old male DBA/1J mice were maintained on either a Trp-sufficient (TS, 0.18% Trp) or Trp-low (TL, 0.05% Trp) diet. CIA was induced by immunization of bovine type II collagen emulsified in CFA at days 0 and 21. 10 mM indole or vehicle was gavaged in TL mice every other day starting at day 0. In some experiments, 100µg of anti-IL23p19 or isotype was administered on days 0, 7, 14, and 21. CIA severity was assessed from days 21-35+, and on day 35 tissues were analyzed by flow cytometry and multiplex immunoassay. GM-CSF-derived bone marrow dendritic cells (BMDCs) were stimulated with LPS ± 1 mM indole for 24hr. Healthy human colon tissue was homogenized and stimulated with 1mM indole for 4hr, and RNA was isolated from flow-sorted CD3+ T cells and CD19+ B cells for RNAseq. Differentially expressed pathways were identified with Ingenuity Pathway Analysis.
Results: We first tested the effect of indole on Th17-promoting cytokines and Th17 cells in CIA and observed significant increases in serum IL-6, TNF, and IL-1β and splenic Th17 cells in TL + Indole mice compared to TL + Vehicle (Fig. 1A-D). We then tested the requirement for IL-23, which promotes pathogenic Th17 differentiation, and found that administration of anti-IL-23 significantly reduced CIA severity in TL + Indole mice (Fig. 1E). To investigate the source of IL-23 and other Th17-promoting cytokines, we stimulated BMDCs with LPS ± indole and saw significant increases in IL-6, TNF, IL-1β, and IL-23 with indole + LPS compared to LPS alone (Table 1). Finally, we tested the effect of indole on human colon lymphocytes and saw increased expression of genes involved in the IL-17 signaling pathway in indole-stimulated B cells (93/187 genes, p=0.02, z=3.87) and T cells (100/187 genes, p=0.02, z=4.36) compared to unstimulated controls (Fig. 2).
Conclusion: Altogether, we show that indole enhances production of Th17-promoting cytokines IL-6, IL-1β, and TNF in BMDCs and in the serum of TL + indole CIA mice. While IL-23 was undetectable in the serum, it was significantly elevated in indole + LPS BMDCs, and treatment with anti-IL-23 reduced CIA severity in TL + Indole mice. Future studies will identify Th17-promoting DC subsets in TL + Indole mice. Finally, enhanced IL-17 signaling in human colon lymphocytes provides proof of concept that indole enhances the Th17 pathway in both mouse and human, providing an opportunity to define and intervene in this pathway to block SpA development.
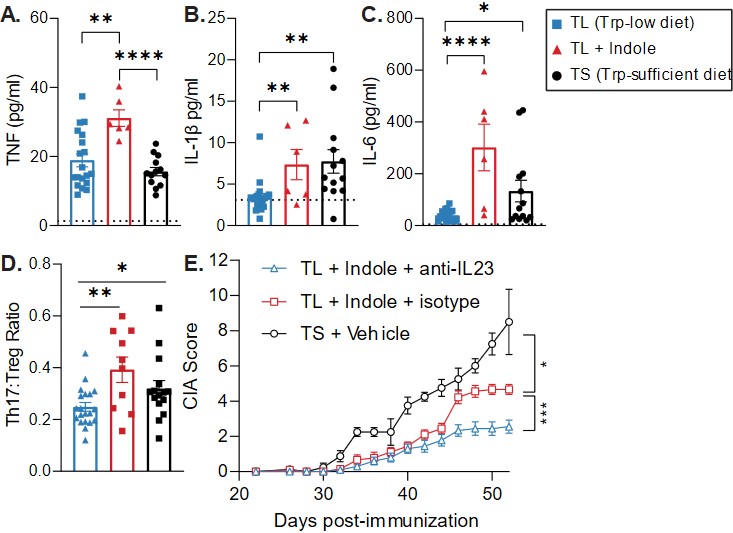 Figure 1. The role of Th17-promoting cytokines in CIA. CIA was induced in TL + vehicle, TL + indole, or TS + vehicle mice (n=6-20). On day 35, serum cytokines were assessed by multiplex immunoassay (A-C) and splenic RORγt+ Th17 cells and FoxP3+ Tregs were quantified by flow cytometry (D). Alternatively, anti-IL23 or isotype were administered to TL + Indole mice (n=10) and disease severity was assessed through day 52.
Figure 1. The role of Th17-promoting cytokines in CIA. CIA was induced in TL + vehicle, TL + indole, or TS + vehicle mice (n=6-20). On day 35, serum cytokines were assessed by multiplex immunoassay (A-C) and splenic RORγt+ Th17 cells and FoxP3+ Tregs were quantified by flow cytometry (D). Alternatively, anti-IL23 or isotype were administered to TL + Indole mice (n=10) and disease severity was assessed through day 52.
.jpg) Table 1. GM-CSF-derived BMDCs were stimulated with LPS +/- indole for 24hr. Supernatant was analyzed by ELISA and RNA was analyzed by RT-qPCR.
Table 1. GM-CSF-derived BMDCs were stimulated with LPS +/- indole for 24hr. Supernatant was analyzed by ELISA and RNA was analyzed by RT-qPCR.
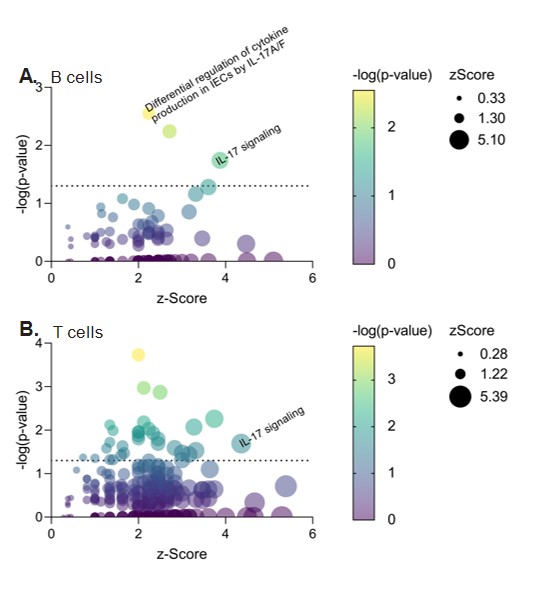 Figure 2. Healthy human colon tissue was homogenized and stimulated with 1mM indole for 4hr; CD3+ T cells and CD19+ B cells were flow sorted and RNA was harvested for RNAseq. IPA pathway analysis revealed differentially expressed pathways.
Figure 2. Healthy human colon tissue was homogenized and stimulated with 1mM indole for 4hr; CD3+ T cells and CD19+ B cells were flow sorted and RNA was harvested for RNAseq. IPA pathway analysis revealed differentially expressed pathways.
Disclosures: B. Seymour, None; B. Trent, None; B. Allen, None; A. Berlinberg, None; K. Kuhn, None.
Background/Purpose: While intestinal dysbiosis and the Th17 pathway are linked to the pathophysiology of spondyloarthritis (SpA), the mechanisms by which this occurs are unknown. We previously linked through microbiome metagenome and intestinal metabolome analyses the presence of indoles derived from bacterial metabolism of tryptophan (Trp) in patients with SpA. We then utilized the collagen-induced arthritis (CIA) model to investigate the role of indole in inflammatory arthritis because the Th17 pathway is required for CIA. We demonstrated that both the microbiome and dietary Trp are required for CIA, and that indole supplementation in either a Trp-deficient or microbiome-deficient setting fulfills this requirement. This led us to hypothesize that indole promotes inflammatory arthritis through enhanced Th17 immunity.
Methods: 6wk-old male DBA/1J mice were maintained on either a Trp-sufficient (TS, 0.18% Trp) or Trp-low (TL, 0.05% Trp) diet. CIA was induced by immunization of bovine type II collagen emulsified in CFA at days 0 and 21. 10 mM indole or vehicle was gavaged in TL mice every other day starting at day 0. In some experiments, 100µg of anti-IL23p19 or isotype was administered on days 0, 7, 14, and 21. CIA severity was assessed from days 21-35+, and on day 35 tissues were analyzed by flow cytometry and multiplex immunoassay. GM-CSF-derived bone marrow dendritic cells (BMDCs) were stimulated with LPS ± 1 mM indole for 24hr. Healthy human colon tissue was homogenized and stimulated with 1mM indole for 4hr, and RNA was isolated from flow-sorted CD3+ T cells and CD19+ B cells for RNAseq. Differentially expressed pathways were identified with Ingenuity Pathway Analysis.
Results: We first tested the effect of indole on Th17-promoting cytokines and Th17 cells in CIA and observed significant increases in serum IL-6, TNF, and IL-1β and splenic Th17 cells in TL + Indole mice compared to TL + Vehicle (Fig. 1A-D). We then tested the requirement for IL-23, which promotes pathogenic Th17 differentiation, and found that administration of anti-IL-23 significantly reduced CIA severity in TL + Indole mice (Fig. 1E). To investigate the source of IL-23 and other Th17-promoting cytokines, we stimulated BMDCs with LPS ± indole and saw significant increases in IL-6, TNF, IL-1β, and IL-23 with indole + LPS compared to LPS alone (Table 1). Finally, we tested the effect of indole on human colon lymphocytes and saw increased expression of genes involved in the IL-17 signaling pathway in indole-stimulated B cells (93/187 genes, p=0.02, z=3.87) and T cells (100/187 genes, p=0.02, z=4.36) compared to unstimulated controls (Fig. 2).
Conclusion: Altogether, we show that indole enhances production of Th17-promoting cytokines IL-6, IL-1β, and TNF in BMDCs and in the serum of TL + indole CIA mice. While IL-23 was undetectable in the serum, it was significantly elevated in indole + LPS BMDCs, and treatment with anti-IL-23 reduced CIA severity in TL + Indole mice. Future studies will identify Th17-promoting DC subsets in TL + Indole mice. Finally, enhanced IL-17 signaling in human colon lymphocytes provides proof of concept that indole enhances the Th17 pathway in both mouse and human, providing an opportunity to define and intervene in this pathway to block SpA development.
 Figure 1. The role of Th17-promoting cytokines in CIA. CIA was induced in TL + vehicle, TL + indole, or TS + vehicle mice (n=6-20). On day 35, serum cytokines were assessed by multiplex immunoassay (A-C) and splenic RORγt+ Th17 cells and FoxP3+ Tregs were quantified by flow cytometry (D). Alternatively, anti-IL23 or isotype were administered to TL + Indole mice (n=10) and disease severity was assessed through day 52.
Figure 1. The role of Th17-promoting cytokines in CIA. CIA was induced in TL + vehicle, TL + indole, or TS + vehicle mice (n=6-20). On day 35, serum cytokines were assessed by multiplex immunoassay (A-C) and splenic RORγt+ Th17 cells and FoxP3+ Tregs were quantified by flow cytometry (D). Alternatively, anti-IL23 or isotype were administered to TL + Indole mice (n=10) and disease severity was assessed through day 52. .jpg) Table 1. GM-CSF-derived BMDCs were stimulated with LPS +/- indole for 24hr. Supernatant was analyzed by ELISA and RNA was analyzed by RT-qPCR.
Table 1. GM-CSF-derived BMDCs were stimulated with LPS +/- indole for 24hr. Supernatant was analyzed by ELISA and RNA was analyzed by RT-qPCR. Figure 2. Healthy human colon tissue was homogenized and stimulated with 1mM indole for 4hr; CD3+ T cells and CD19+ B cells were flow sorted and RNA was harvested for RNAseq. IPA pathway analysis revealed differentially expressed pathways.
Figure 2. Healthy human colon tissue was homogenized and stimulated with 1mM indole for 4hr; CD3+ T cells and CD19+ B cells were flow sorted and RNA was harvested for RNAseq. IPA pathway analysis revealed differentially expressed pathways.Disclosures: B. Seymour, None; B. Trent, None; B. Allen, None; A. Berlinberg, None; K. Kuhn, None.

