Back
Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Treatment I: Switching or Discontinuation of Therapies (1585–1590
1586: Sustained Remission Following the Discontinuation of Tofacitinib in Patients with Rheumatoid Arthritis (XANADU Study): A Multicenter, Prospective, and Randomized Controlled Study
Sunday, November 13, 2022
3:15 PM – 3:25 PM Eastern Time
Location: Room 120
- SK
Satoshi Kubo, MD, PhD
Department of Molecular Targeted Therapies (DMTT), University of Occupational and Environmental Health, Japan
Kitakyushu, Japan
Presenting Author(s)
Satoshi Kubo1, Yusuke Miyazaki2, Koichi Amano3, Kiyoshi Matsui4, Hideto Kameda5, Yoshino Inoue6, Shingo Nakayamada6, Takehisa Ogura7, Yuko Kaneko8, Kunihiro Yamaoka9 and Yoshiya Tanaka10, 1Department of Advanced Targeted Therapies, University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 2The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Kiakyusyu Fukuoka, Japan, 3Saitama Medical Center, Kawagoe, Japan, 4Hyogo College of Medicine, Nishinomiya, Japan, 5Toho University, Tokyo, Japan, 6First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 7Toho university, Meguro-ku, Japan, 8Keio University, Tokyo, Japan, 9Department of Rheumatology and Infectious Diseases, Kitasato University School of Medicine, Kanagawa, Japan, 10University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan
Background/Purpose: To investigate sustained remission following the discontinuation of tofacitinib in patients with rheumatoid arthritis (RA).
Methods: RA patients who had an inadequate response to methotrexate (MTX-IR) with or without bDMARDs were randomly divided (1:1) into two groups at baseline, and tofacitinib treatment in combination with MTX was administered to both groups. Either MTX or tofacitinib was then withdrawn if patients achieved CDAI remission at week 52 and patients were followed up with periodically. The primary outcome was the proportion of patients who sustained clinical remission at week 104. The secondary endpoints were the proportion of patients who had severe adverse events (SAE) throughout the study and the proportion of patients who achieved clinical remission after rescue by re-administration of tofacitinib or MTX.
Results: A total of 113 patients (56 with tofacitinib withdrawal and 57 with MTX withdrawal) participated in this study. Among them, a total of 48 patients achieved remission at week 52. After discontinuation of tofacitinib, 29.2% of patients remained in remission and 41.7% maintained low disease activity (Fig. 1A). In contrast, 50.0% of patients sustained in remission and 65.0% maintained low disease activity in the MTX discontinuation group (Fig. 1B). When we compared the two groups, the CDAI remission and low disease activity rate were numerically higher in the MTX discontinuation group compared to the tofacitinib discontinuation group, but the difference was not statistically significant at week 104 (p=0.1576 and p=0.1228, respectively). A greater proportion of bio-naïve patients achieved remission at week 52 and sustained low disease activity with tofacitinib discontinuation at week 104 (Fig. 2A and B). Additionally, the patients who were able to discontinue tofacitinib without flares had lower rheumatoid factor (RF) (p=0.04) and anti-CCP antibody (p=0.051) before discontinuation of tofacitinib. This trend was not observed in the MTX discontinuation group, and lower CDAI at the drug discontinuation was the main factor to the successful discontinuation of MTX. ROC curve analysis revealed the cutoff value of RF contributing to the sustained low disease activity after tofacitinib discontinuation as 56 U/ml (sensitivity 0.8, specificity 0.85). When the patients in the tofacitinib discontinuation group were divided into two groups according to the RF value, 66.7% of patients with a lower RF remained in remission after withdrawal (Fig. 3A and B). A total of 8 SAEs occurred before week 52, while no SAEs were recorded after tofacitinib discontinuation. In patients who relapsed after tofacitinib discontinuation, 71.4% of patients achieved remission with resumption of tofacitinib.
Conclusion: This study is the first multicenter, prospective, open label, and randomized controlled trial to investigate sustained remission following the discontinuation of tofacitinib. We showed that the discontinuation of JAK inhibitors is feasible, but not the universal best option. However, post-hoc analysis revealed that successful withdrawal is possible if patients are appropriately selected. In particular, no or low titer seropositivity can contribute to the success of drug withdrawal.
.jpg) Figure 1. Sankey diagram describing proportion of patients per CDAI disease activity category over time. (A) Tofacitinib discontinuation arm (n=56). (B) Methotrexate discontinuation arm (n=57). Data are n (%).
Figure 1. Sankey diagram describing proportion of patients per CDAI disease activity category over time. (A) Tofacitinib discontinuation arm (n=56). (B) Methotrexate discontinuation arm (n=57). Data are n (%).
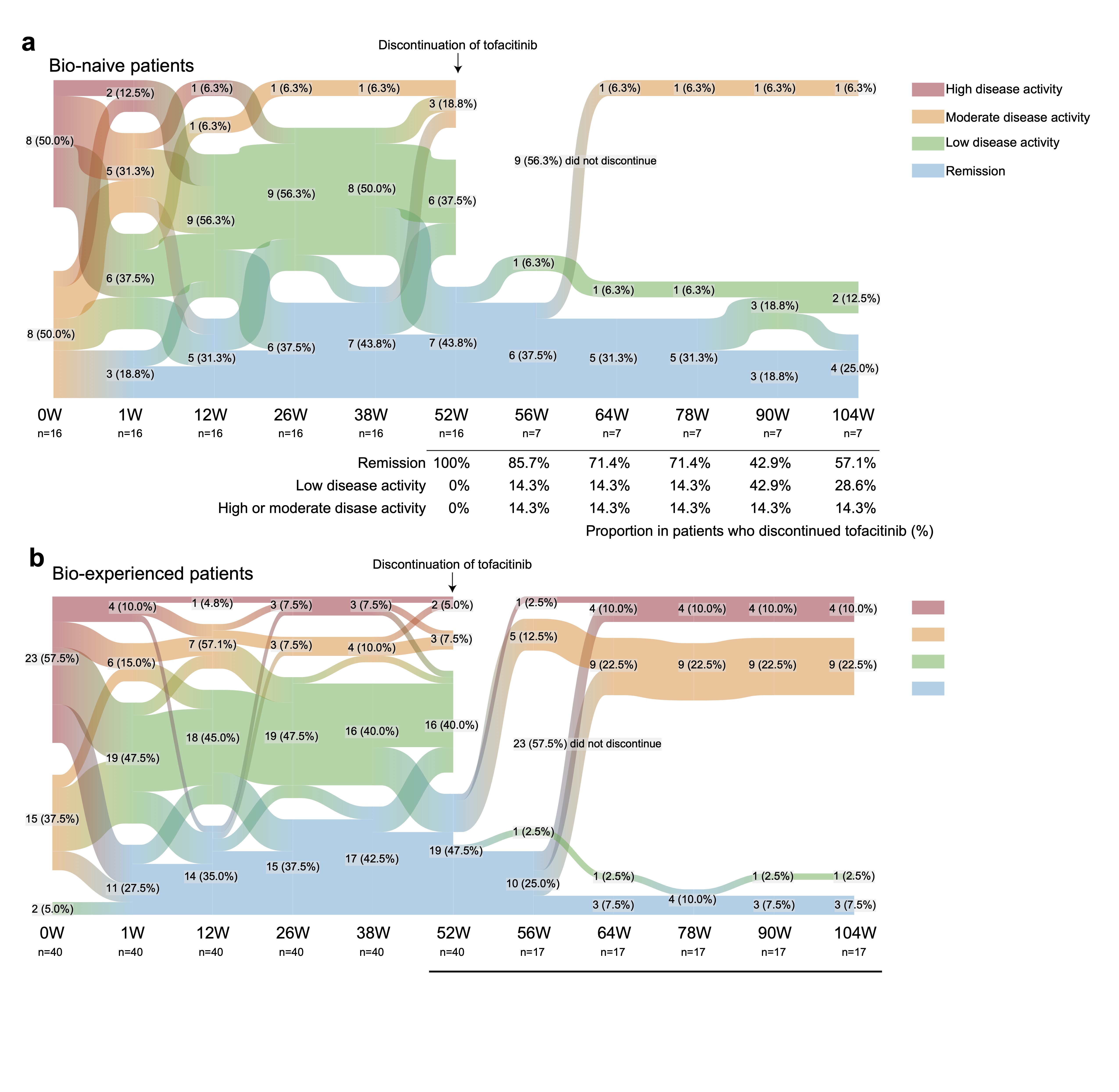 Figure 2. Sankey diagram describing proportion of patients per CDAI disease activity category over time in the subgroup. (A) Bio-naïve patients (n=16). (B) Bio-experienced patients (n=21). Data are n (%).
Figure 2. Sankey diagram describing proportion of patients per CDAI disease activity category over time in the subgroup. (A) Bio-naïve patients (n=16). (B) Bio-experienced patients (n=21). Data are n (%).
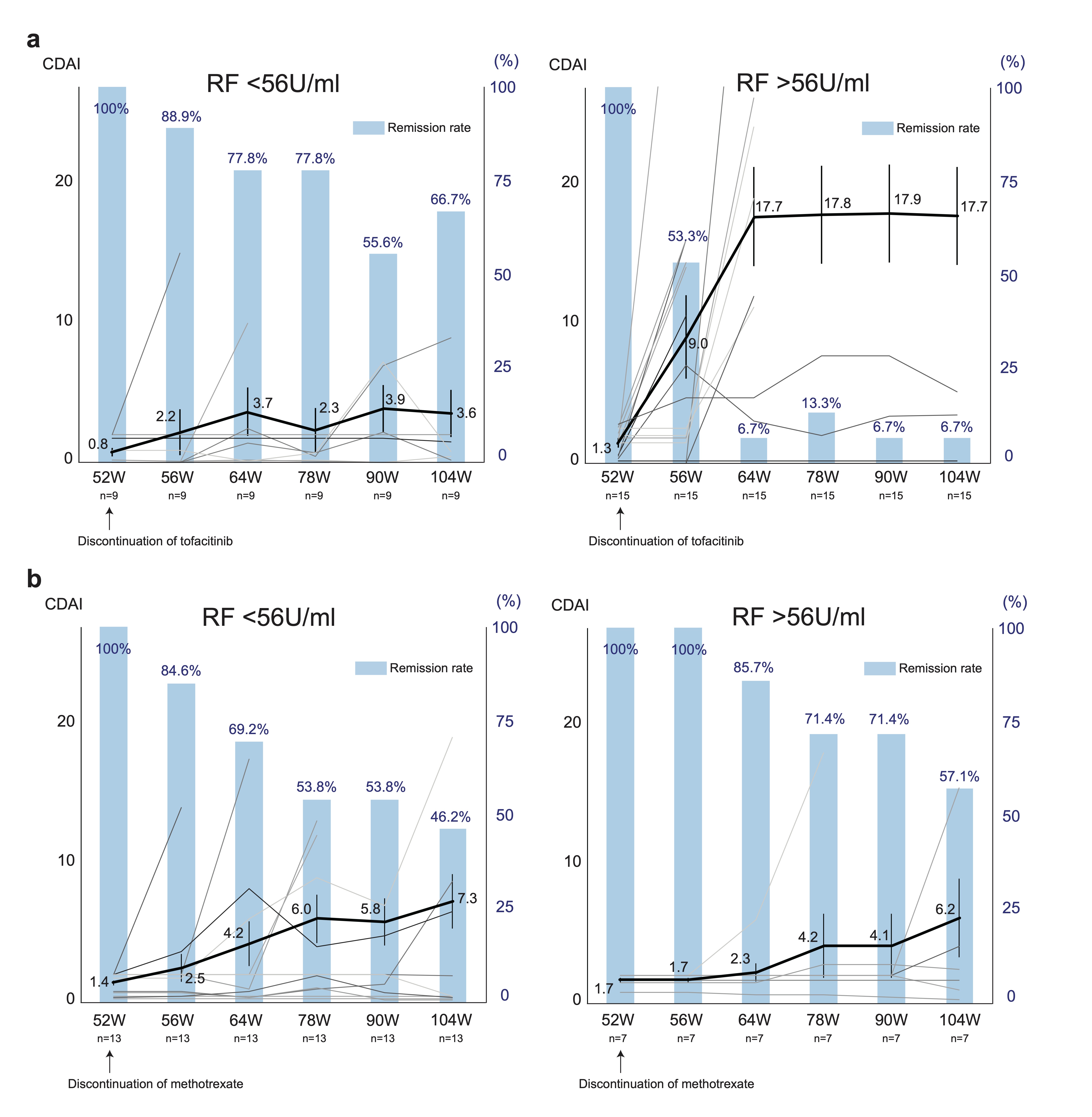 Figure 3. Serial changes in the CDAI and clinical remission according to CDAI after discontinuation of tofacitinib or methotrexate in the subgroup. Spaghetti plot (black lines: mean value, grey lines:of the values of individual patients) and bar graph of remission rate are shown. (A) RF lower titer group (left) and higher titer group (right) in the tofacitinib discontinuation arm. (B) RF lower titer group (left) and higher titer group (right) in the methotrexate discontinuation arm. Data are mean (SEM) and %.
Figure 3. Serial changes in the CDAI and clinical remission according to CDAI after discontinuation of tofacitinib or methotrexate in the subgroup. Spaghetti plot (black lines: mean value, grey lines:of the values of individual patients) and bar graph of remission rate are shown. (A) RF lower titer group (left) and higher titer group (right) in the tofacitinib discontinuation arm. (B) RF lower titer group (left) and higher titer group (right) in the methotrexate discontinuation arm. Data are mean (SEM) and %.
Disclosures: S. Kubo, None; Y. Miyazaki, None; K. Amano, Asahi-Kasei, AbbVie/Abbott, Chugai Pharmaceutical, Eisai, Eli Lilly, Pfizer; K. Matsui, None; H. Kameda, AbbVie, Asahi-Kasei, Lilly, Janssen, Novartis, Sanofi, Bristol-Myers Squibb, Chugai, Mitsubishi Tanabe, Eisai, Astellas, Gilead, Taisho; Y. Inoue, None; S. Nakayamada, None; T. Ogura, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, Janssen, Eisai, Asahi kasei; Y. Kaneko, Pfizer; K. Yamaoka, AbbVie GK, Astellas, BMS, Chugai, Mitsubishi-Tanabe, Pfizer, Takeda; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly.
Background/Purpose: To investigate sustained remission following the discontinuation of tofacitinib in patients with rheumatoid arthritis (RA).
Methods: RA patients who had an inadequate response to methotrexate (MTX-IR) with or without bDMARDs were randomly divided (1:1) into two groups at baseline, and tofacitinib treatment in combination with MTX was administered to both groups. Either MTX or tofacitinib was then withdrawn if patients achieved CDAI remission at week 52 and patients were followed up with periodically. The primary outcome was the proportion of patients who sustained clinical remission at week 104. The secondary endpoints were the proportion of patients who had severe adverse events (SAE) throughout the study and the proportion of patients who achieved clinical remission after rescue by re-administration of tofacitinib or MTX.
Results: A total of 113 patients (56 with tofacitinib withdrawal and 57 with MTX withdrawal) participated in this study. Among them, a total of 48 patients achieved remission at week 52. After discontinuation of tofacitinib, 29.2% of patients remained in remission and 41.7% maintained low disease activity (Fig. 1A). In contrast, 50.0% of patients sustained in remission and 65.0% maintained low disease activity in the MTX discontinuation group (Fig. 1B). When we compared the two groups, the CDAI remission and low disease activity rate were numerically higher in the MTX discontinuation group compared to the tofacitinib discontinuation group, but the difference was not statistically significant at week 104 (p=0.1576 and p=0.1228, respectively). A greater proportion of bio-naïve patients achieved remission at week 52 and sustained low disease activity with tofacitinib discontinuation at week 104 (Fig. 2A and B). Additionally, the patients who were able to discontinue tofacitinib without flares had lower rheumatoid factor (RF) (p=0.04) and anti-CCP antibody (p=0.051) before discontinuation of tofacitinib. This trend was not observed in the MTX discontinuation group, and lower CDAI at the drug discontinuation was the main factor to the successful discontinuation of MTX. ROC curve analysis revealed the cutoff value of RF contributing to the sustained low disease activity after tofacitinib discontinuation as 56 U/ml (sensitivity 0.8, specificity 0.85). When the patients in the tofacitinib discontinuation group were divided into two groups according to the RF value, 66.7% of patients with a lower RF remained in remission after withdrawal (Fig. 3A and B). A total of 8 SAEs occurred before week 52, while no SAEs were recorded after tofacitinib discontinuation. In patients who relapsed after tofacitinib discontinuation, 71.4% of patients achieved remission with resumption of tofacitinib.
Conclusion: This study is the first multicenter, prospective, open label, and randomized controlled trial to investigate sustained remission following the discontinuation of tofacitinib. We showed that the discontinuation of JAK inhibitors is feasible, but not the universal best option. However, post-hoc analysis revealed that successful withdrawal is possible if patients are appropriately selected. In particular, no or low titer seropositivity can contribute to the success of drug withdrawal.
.jpg) Figure 1. Sankey diagram describing proportion of patients per CDAI disease activity category over time. (A) Tofacitinib discontinuation arm (n=56). (B) Methotrexate discontinuation arm (n=57). Data are n (%).
Figure 1. Sankey diagram describing proportion of patients per CDAI disease activity category over time. (A) Tofacitinib discontinuation arm (n=56). (B) Methotrexate discontinuation arm (n=57). Data are n (%).  Figure 2. Sankey diagram describing proportion of patients per CDAI disease activity category over time in the subgroup. (A) Bio-naïve patients (n=16). (B) Bio-experienced patients (n=21). Data are n (%).
Figure 2. Sankey diagram describing proportion of patients per CDAI disease activity category over time in the subgroup. (A) Bio-naïve patients (n=16). (B) Bio-experienced patients (n=21). Data are n (%). Figure 3. Serial changes in the CDAI and clinical remission according to CDAI after discontinuation of tofacitinib or methotrexate in the subgroup. Spaghetti plot (black lines: mean value, grey lines:of the values of individual patients) and bar graph of remission rate are shown. (A) RF lower titer group (left) and higher titer group (right) in the tofacitinib discontinuation arm. (B) RF lower titer group (left) and higher titer group (right) in the methotrexate discontinuation arm. Data are mean (SEM) and %.
Figure 3. Serial changes in the CDAI and clinical remission according to CDAI after discontinuation of tofacitinib or methotrexate in the subgroup. Spaghetti plot (black lines: mean value, grey lines:of the values of individual patients) and bar graph of remission rate are shown. (A) RF lower titer group (left) and higher titer group (right) in the tofacitinib discontinuation arm. (B) RF lower titer group (left) and higher titer group (right) in the methotrexate discontinuation arm. Data are mean (SEM) and %.Disclosures: S. Kubo, None; Y. Miyazaki, None; K. Amano, Asahi-Kasei, AbbVie/Abbott, Chugai Pharmaceutical, Eisai, Eli Lilly, Pfizer; K. Matsui, None; H. Kameda, AbbVie, Asahi-Kasei, Lilly, Janssen, Novartis, Sanofi, Bristol-Myers Squibb, Chugai, Mitsubishi Tanabe, Eisai, Astellas, Gilead, Taisho; Y. Inoue, None; S. Nakayamada, None; T. Ogura, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, Janssen, Eisai, Asahi kasei; Y. Kaneko, Pfizer; K. Yamaoka, AbbVie GK, Astellas, BMS, Chugai, Mitsubishi-Tanabe, Pfizer, Takeda; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly.

