Back
Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, and Outcomes II: Complications (1591–1596)
1595: Association of Sustained Lupus Low Disease Activity State with Improved Outcomes in SLE: A Multinational Prospective Cohort Study
Sunday, November 13, 2022
4:00 PM – 4:10 PM Eastern Time
Location: Room 204
.png)
Eric Morand, MD, PhD
Monash University
Melbourne, Victoria, Australia
Presenting Author(s)
Vera Golder1, Rangi Kandane-Rathnayake1, Ning Li1, Worawit Louthrenoo2, Yi-Hsing Chen3, Jiacai Cho4, Aisha Lateef5, Laniyati Hamijoyo6, Luo Shue Fen7, Yeong-Jian Wu7, Sandra Navarra8, Leonid Zamora8, Zhanguo Li9, An Yuan10, Sargunan Sockalingam11, Yasuhiro Katsumata12, Masayoshi Harigai12, Yanjie Hao13, Zhouli Zhang14, Duminda Basnayake15, Madelynn Chan16, Jun Kikuchi17, Tsutomu Takeuchi18, Sang-Cheol Bae19, Fiona Goldblatt20, Shereen Oon21, Sean O'Neill22, Kathryn Gibson22, Kristine Ng23, Hui Nee Annie Law24, Nicole Tugnet25, Sunil Kumar26, Cherica Tee27, Michael Tee27, Yoshiya Tanaka28, Chak Sing29, Alberta Hoi30, Mandana Nikpour31 and Eric Morand32, 1Monash University, Clayton, Australia, 2Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand, 3Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan, 4National University Health System (NUHS), Singapore, Singapore, 5National University Hospital, Singapore, Singapore, 6Hasan Sadikin Hospital, Jakarta Selatan, Indonesia, 7Chang Gung Memorial Hospital, Taoyuan, Taiwan, 8University of Santo Tomas, Manila, Philippines, 9Peking University People's Hospital, Beijing, China, 10Peking University Health Science Center, Beijing, China, 11University of Malaya, Kuala Lumpur, Kuala Lumpur, Malaysia, 12Division of Rheumatology, Department of Internal Medicine, Tokyo Women’s Medical University School of Medicine, Tokyo, Japan, 13The University of Melbourne, Melbourne, Australia, 14Peking University First Hospital, Beijing, China, 15Teaching Hospital Kandy, Kandy, Sri Lanka, 16Tan Tock Seng Hospital, Singapore, Singapore, 17Keio University School of Medicine, Tokyo, Japan, 18Keio University and Saitama Medical University, Tokyo, Japan, 19Hanyang University Medical Center, Seoul, Republic of Korea, 20Flinders Medical Centre, Adelaide, Australia, 21St Vincent's Hospital, Fitzroy, Australia, 22Liverpool Hospital, Sydney, Australia, 23North Shore Hospital, Auckland, New Zealand, 24Department of Rheumatology and Immunology, Singapore General Hospital, Singapore, Singapore, 25Greenlane Clinical Centre, Auckland, New Zealand, 26Middlemore Hospital, Auckland, New Zealand, 27University of the Philippines, Quezon City, Philippines, 28University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 29The University of Hong Kong, Pok Fu Lam, Hong Kong, 30Monash Health, Melbourne, Australia, 31The University of Melbourne at St. Vincent's Hospital Melbourne, Melbourne, Australia, 32Monash University, Victoria; Department of Rheumatology, Monash Health, Melbourne, Australia
Background/Purpose: Since the initial prospective validation of the Lupus Low Disease Activity State (LLDAS), this treat-to-target endpoint has been studied in numerous other cohorts, with results supporting its protective associations against flare and damage accrual. The mean duration of follow up in the original prospective validation was 2 years, potentially impacting on the ability to detect longer term signals in disease flare and irreversible damage, which typically accrues slowly. In this study we aimed to assess long-term associations of sustained LLDAS with protection from damage accrual and flare.
Methods: Adult SLE patients were recruited and followed prospectively from May 2013 to Dec 2020. Patients with ≥2 visits and no missing data were included in analysis. Multi failure time-to-event (Cox regression) analyses were used to assess the impact of sustained LLDAS on irreversible damage accrual (SLICC damage index) and flare (SELENA flare index), with dose and threshold effects studied. DORIS Remission was similarly assessed.
Results: 3,770 SLE patients were followed for (mean ± SD) 3.1 ± 2.4 years, totalling 37,834 visits. Most patients (n 3,128, 76.2%) attained LLDAS on at least one occasion. Any single visit in LLDAS was associated with significant protection against subsequent damage accrual (HR 0.60, 95%CI 0.52-0.69, p< 0.001) and flare (HR 0.57, 95%CI 0.53-0.61, p< 0.001). 2,699 (71.6%) sustained LLDAS for >3 months. Increasing durations of sustained LLDAS from >3 to >24 months corresponded to increased protective effects against damage and flare, whether measured as a dose effect or as a threshold (Table 1). LLDAS was more attainable compared to Remission (61.7% of patients ever), whilst conferring a similar magnitude of protection against damage and flare for sustained time ( >3 months Remission: HR 0.69, 95%CI 0.60-0.80, p< 0.001, and HR 0.65, 95%CI 0.60-0.71, p< 0.001 respectively).
Conclusion: In this long term prospective cohort study, we confirm significant protective effects of LLDAS against damage accrual and flare, as well as demonstrating deepening protection with longer durations of sustained LLDAS. LLDAS is a more attainable treat-to-target endpoint than remission while having similar protective effects.
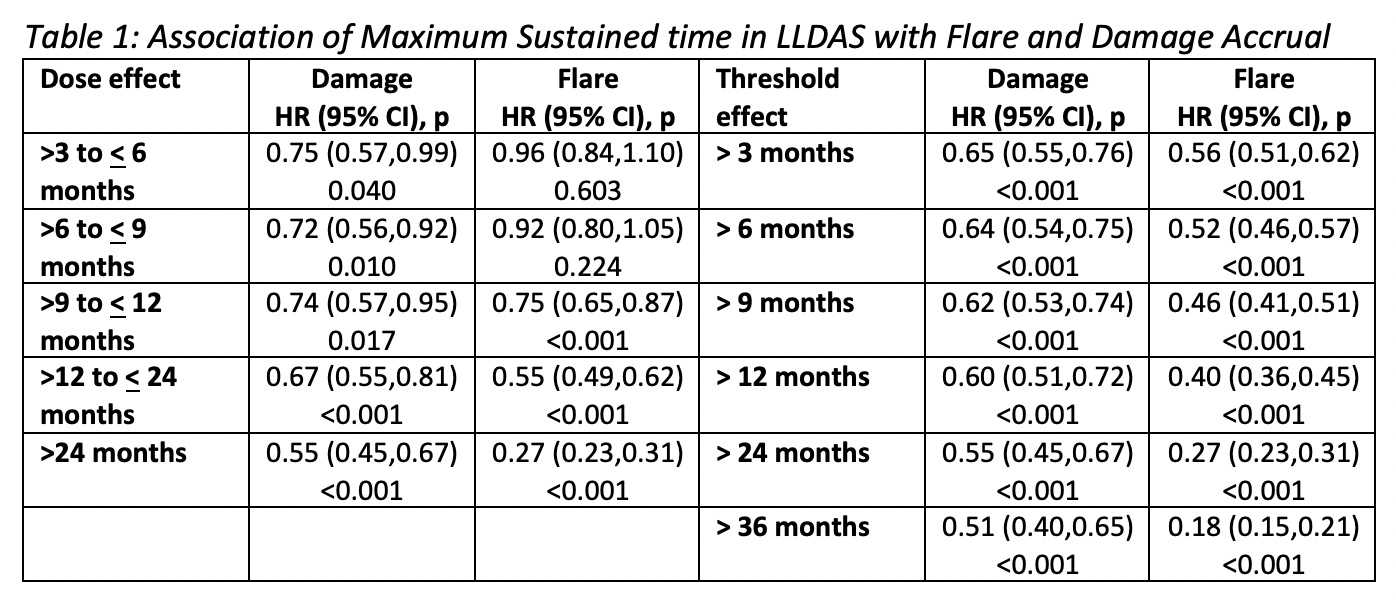 Dose effect – groups mutually exclusive;
Dose effect – groups mutually exclusive;
Threshold effect – increasing threshold of sustained time;
Patients grouped based on maximum sustained time per patient
Disclosures: V. Golder, None; R. Kandane-Rathnayake, None; N. Li, None; W. Louthrenoo, None; Y. Chen, AbbVie, AstraZeneca, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharma Taiwan, Inova Diagnostics, Eli Lilly, Johnson & Johnson, GlaxoSmithKline, MSD, Novartis, Roche, Sanofi, Thermo Fisher Scientific, UCB, United Biopharma, Agnitio Science & Technology, Boehringer Ingelheim, National Yang-Ming University, Pfizer Inc, Taiwan Ministry of Science and Technology, Taiwan Department of Health and Welfare, Taichung Veterans General Hospital and UCB, Gilead Sciences, Guigai; J. Cho, None; A. Lateef, None; L. Hamijoyo, None; L. Fen, None; Y. Wu, None; S. Navarra, Biogen, Astellas, Janssen, Novartis, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline (GSK); L. Zamora, None; Z. Li, Pfizer, Eli Lilly, Novartis, GlaxoSmithKlein(GSK), AbbVie/Abbott, Roche; A. Yuan, None; S. Sockalingam, Pfizer, Roche, Novartis; Y. Katsumata, GlaxoSmithKline K.K., AstraZeneca K.K., Pfizer Japan Inc., Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co., Ltd., Asahi Kasei Pharma, Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc.; M. Harigai, AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., Viatris Japan; Y. Hao, None; Z. Zhang, None; D. Basnayake, None; M. Chan, None; J. Kikuchi, None; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi; S. Bae, None; F. Goldblatt, None; S. Oon, None; S. O'Neill, None; K. Gibson, Eli Lilly Pty Ltd; K. Ng, None; H. Law, None; N. Tugnet, None; S. Kumar, None; C. Tee, None; M. Tee, None; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; C. Sing, None; A. Hoi, None; M. Nikpour, Janssen, AstraZeneca, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Bristol-Myers Squibb(BMS); E. Morand, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Genentech, GlaxoSmithKlein(GSK), Janssen, Novartis, Servier, UCB, EMD Serono, Billerica, MA, USA.
Background/Purpose: Since the initial prospective validation of the Lupus Low Disease Activity State (LLDAS), this treat-to-target endpoint has been studied in numerous other cohorts, with results supporting its protective associations against flare and damage accrual. The mean duration of follow up in the original prospective validation was 2 years, potentially impacting on the ability to detect longer term signals in disease flare and irreversible damage, which typically accrues slowly. In this study we aimed to assess long-term associations of sustained LLDAS with protection from damage accrual and flare.
Methods: Adult SLE patients were recruited and followed prospectively from May 2013 to Dec 2020. Patients with ≥2 visits and no missing data were included in analysis. Multi failure time-to-event (Cox regression) analyses were used to assess the impact of sustained LLDAS on irreversible damage accrual (SLICC damage index) and flare (SELENA flare index), with dose and threshold effects studied. DORIS Remission was similarly assessed.
Results: 3,770 SLE patients were followed for (mean ± SD) 3.1 ± 2.4 years, totalling 37,834 visits. Most patients (n 3,128, 76.2%) attained LLDAS on at least one occasion. Any single visit in LLDAS was associated with significant protection against subsequent damage accrual (HR 0.60, 95%CI 0.52-0.69, p< 0.001) and flare (HR 0.57, 95%CI 0.53-0.61, p< 0.001). 2,699 (71.6%) sustained LLDAS for >3 months. Increasing durations of sustained LLDAS from >3 to >24 months corresponded to increased protective effects against damage and flare, whether measured as a dose effect or as a threshold (Table 1). LLDAS was more attainable compared to Remission (61.7% of patients ever), whilst conferring a similar magnitude of protection against damage and flare for sustained time ( >3 months Remission: HR 0.69, 95%CI 0.60-0.80, p< 0.001, and HR 0.65, 95%CI 0.60-0.71, p< 0.001 respectively).
Conclusion: In this long term prospective cohort study, we confirm significant protective effects of LLDAS against damage accrual and flare, as well as demonstrating deepening protection with longer durations of sustained LLDAS. LLDAS is a more attainable treat-to-target endpoint than remission while having similar protective effects.
 Dose effect – groups mutually exclusive;
Dose effect – groups mutually exclusive; Threshold effect – increasing threshold of sustained time;
Patients grouped based on maximum sustained time per patient
Disclosures: V. Golder, None; R. Kandane-Rathnayake, None; N. Li, None; W. Louthrenoo, None; Y. Chen, AbbVie, AstraZeneca, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharma Taiwan, Inova Diagnostics, Eli Lilly, Johnson & Johnson, GlaxoSmithKline, MSD, Novartis, Roche, Sanofi, Thermo Fisher Scientific, UCB, United Biopharma, Agnitio Science & Technology, Boehringer Ingelheim, National Yang-Ming University, Pfizer Inc, Taiwan Ministry of Science and Technology, Taiwan Department of Health and Welfare, Taichung Veterans General Hospital and UCB, Gilead Sciences, Guigai; J. Cho, None; A. Lateef, None; L. Hamijoyo, None; L. Fen, None; Y. Wu, None; S. Navarra, Biogen, Astellas, Janssen, Novartis, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline (GSK); L. Zamora, None; Z. Li, Pfizer, Eli Lilly, Novartis, GlaxoSmithKlein(GSK), AbbVie/Abbott, Roche; A. Yuan, None; S. Sockalingam, Pfizer, Roche, Novartis; Y. Katsumata, GlaxoSmithKline K.K., AstraZeneca K.K., Pfizer Japan Inc., Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co., Ltd., Asahi Kasei Pharma, Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc.; M. Harigai, AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., Viatris Japan; Y. Hao, None; Z. Zhang, None; D. Basnayake, None; M. Chan, None; J. Kikuchi, None; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi; S. Bae, None; F. Goldblatt, None; S. Oon, None; S. O'Neill, None; K. Gibson, Eli Lilly Pty Ltd; K. Ng, None; H. Law, None; N. Tugnet, None; S. Kumar, None; C. Tee, None; M. Tee, None; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; C. Sing, None; A. Hoi, None; M. Nikpour, Janssen, AstraZeneca, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Bristol-Myers Squibb(BMS); E. Morand, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Genentech, GlaxoSmithKlein(GSK), Janssen, Novartis, Servier, UCB, EMD Serono, Billerica, MA, USA.

