Back
Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Treatment I: Switching or Discontinuation of Therapies (1585–1590
1590: Pain and Treatment Switching Among Patients in the CorEvitasTM Rheumatoid Arthritis Registry
Sunday, November 13, 2022
4:15 PM – 4:25 PM Eastern Time
Location: Room 120

Joshua Baker, MD, MS
University of Pennsylvania
Philadelphia, PA, United States
Presenting Author(s)
Joshua Baker1, J Morel Symons2, Jud C Janak3, Oksana Pugach3, Elizabeth Kohl3, Miao Yu3, Dave Webb4, Alan A Martin4, Didier Saurigny5 and Marguerite Bracher5, 1University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, 2GlaxoSmithKline, Collegeville, PA, 3CorEvitas, LLC, Waltham, MA, 4GlaxoSmithKline, Brentford, United Kingdom, 5GlaxoSmithKline, Stevenage, United Kingdom
Background/Purpose: For many patients with RA on targeted therapies, the chronic burden of pain leads to therapies being deemed suboptimally effective and may affect treatment decisions. Our objective was to explore the association between patient-reported pain and switching biologic/targeted synthetic (b/ts)DMARDs.
Methods: Patients in the CorEvitas RA registry who met the following criteria were included: started a b/tsDMARD as of 1/1/2012; had ≥2 routine clinical visits; and no b/tsDMARD interruption (defined as being off treatment for >2 months). The outcome of interest was time to treatment switch to a b/tsDMARD with a different MOA. The exposure of interest was time-varying patient-reported pain measured on the visual analog scale (Pain VAS: 0–100 mm): mild (0–44 mm), moderate (45–74 mm), and severe (75–100 mm). Sociodemographics, lifestyle, disease characteristics, treatment history, and patient-reported outcomes were explored as potential confounders. Disease activity was analyzed with both time-varying Clinical Disease Activity Index (CDAI) and modified (m)CDAI (minus patient global assessment and tender joint count). Descriptive statistics stratified by baseline pain category were reported. Hazard ratios (HR) and 95% confidence intervals were reported for unadjusted and adjusted Cox hazards models with cluster-robust standard errors.
Results: Among 7806 patients, 9587 b/tsDMARD starts (Table, Panel B) were included for the following pain categories: mild (n=4126; 43%), moderate (n=2976; 31%), and severe (n=2430; 25%), missing (n=55; 1%). In patients with severe pain vs mild pain (Table, Panel A), a greater proportion were smokers (23% vs 15%); obese (52% vs 40%); and there was a higher prevalence of depression (43% vs 28%) and fibromyalgia (12% vs 3%). A higher proportion with severe pain vs mild pain reported ≥2 previous bDMARDs (50% vs 38%) and use of opioids (49% vs 18%), prednisone (39% vs 26%), and anti-depressants (33% vs 20%). The unadjusted risk of switching b/tsDMARD was ~2 times higher for severe vs mild pain (HR=1.98; 1.60–2.44; Figure). The fully adjusted risk for severe vs mild pain after accounting for confounders (HR=1.37; 1.02–1.86) was similar to the model that only included time-varying CDAI as a confounder (HR=1.35; 1.08–1.69). Compared with models that incorporated only time-varying CDAI, the risk of switching for severe pain was higher when adjusting instead for only time-varying mCDAI (HR=1.51; 1.22–1.87).
Conclusion: Patients that report greater pain are more likely to switch to a b/tsDMARD of a different MOA; an observation that remained after adjusting for confounders, including time-varying disease activity. Overall, these findings suggest that persistent pain is common in RA and influences treatment decisions, particularly a switch to a different MOA. Approaches that reduce chronic pain in this population may help reduce treatment cycling.
This GSK-funded study (214024) was sponsored by CorEvitas, LLC, with editorial support by Fishawack Indicia Ltd, funded by GSK.
.jpg)
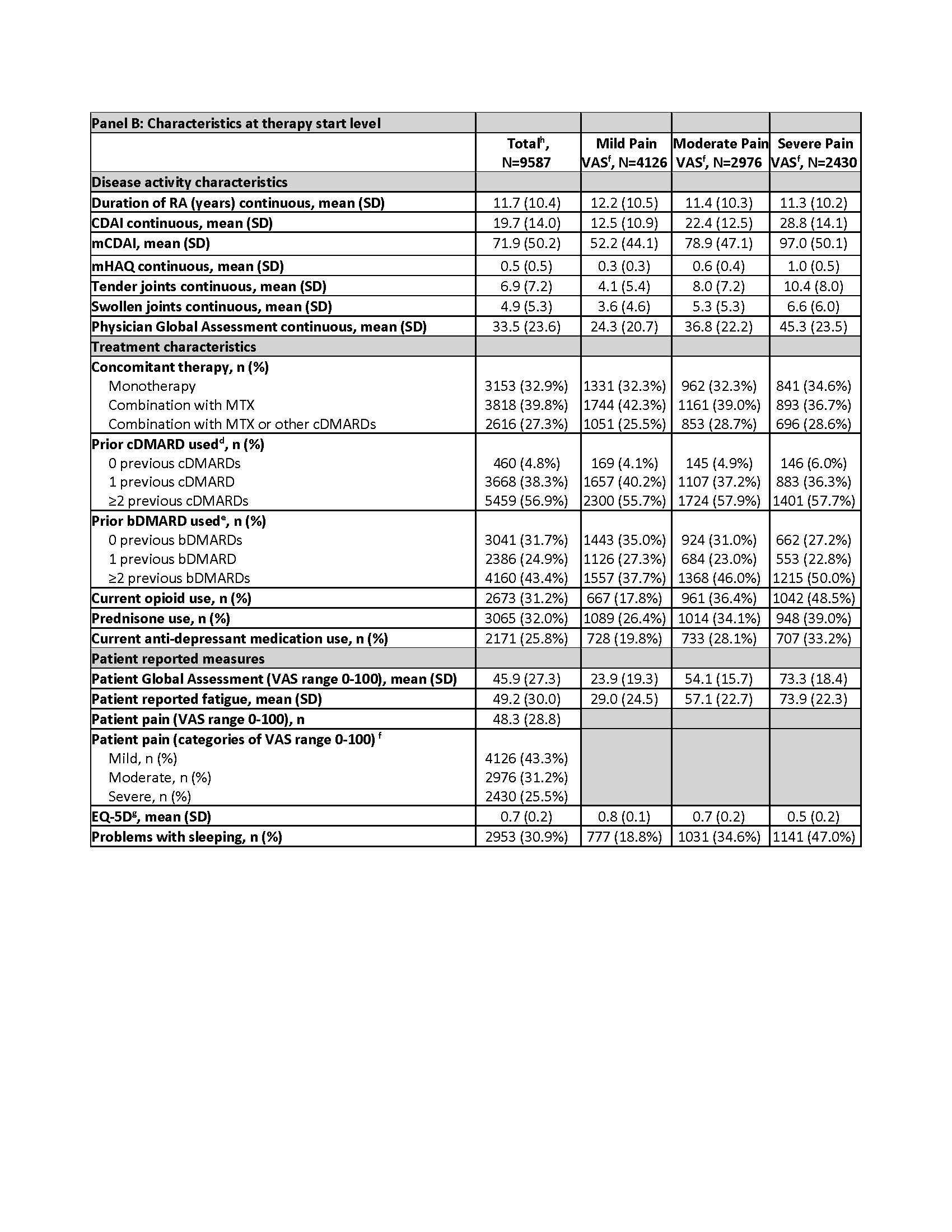 Table. Descriptive characteristics of sociodemographic factors, health behaviors and characteristics, disease, and treatment characteristics at baseline, by total sample and Pain VAS groups.
Table. Descriptive characteristics of sociodemographic factors, health behaviors and characteristics, disease, and treatment characteristics at baseline, by total sample and Pain VAS groups.
a Insurance type is not mutually exclusive
b Based on the Centers for Disease Control and Prevention (CDC) cut-offs for normal/underweight (under 25); overweight (25.0–29.9); and obese (30.0 and above)
c Infections include Joint/bursa, cellulitis/skin, sinusitis, Candida, diverticulitis, sepsis, pneumonia, bronchitis, gastroenteritis, meningitis/encephalitis, urinary tract, upper respiratory, tuberculosis, and other. Infections resulting in hospitalization or administration of IV antibiotics indicates serious infection.
d Prior non-biologic systemic use count does not include the current non-biologic systemic therapy, if applicable
e Prior biologics count does not include subject’s current biologic, biosimilars are not counted
f No pain and mild pain (0–44 mm), moderate pain (45–74 mm), severe pain (75–100 mm)
g The EuroQoL-5 Dimension (EQ-5D) computes an index value using the individual mobility, self-care, usual activities, pain or discomfort, and anxiety or depression responses from the EQ-5D quality-of-life instrument.
h 47 patients (55 therapy starts) had missing baseline pain
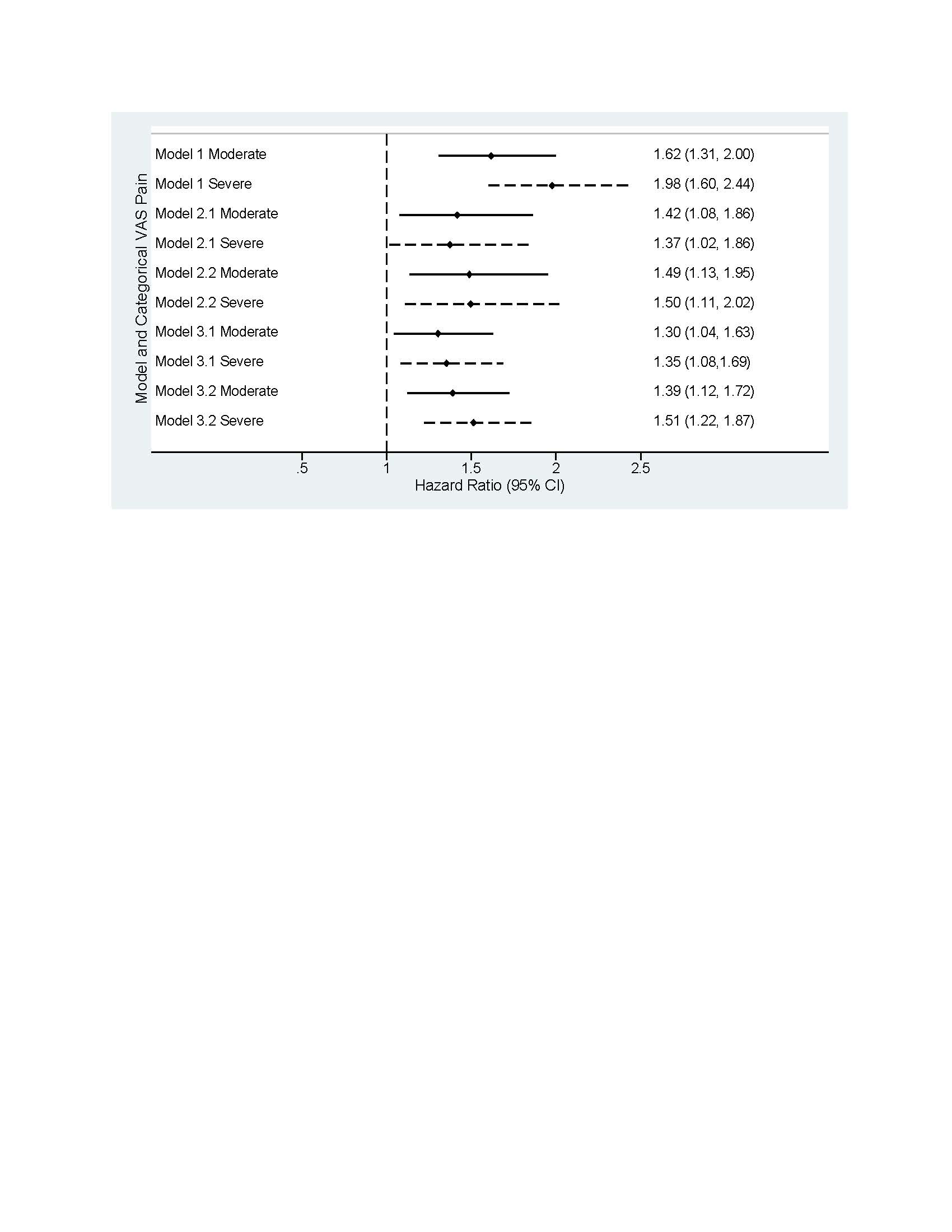 Figure. Forest plots of HR for switching b/tsDMARD with categorical Pain VAS (mild pain as a reference group) across different models.
Figure. Forest plots of HR for switching b/tsDMARD with categorical Pain VAS (mild pain as a reference group) across different models.
Note: Unadjusted model: Pain VAS as only covariate, sample size=9587, number of events=980, mean follow-up time=22.9 months, median follow-up time=15 months
Model 1 - Unadjusted model: time-varying Pain VAS categories as only covariate, low Pain VAS group as a reference
Model 2.1 - Fully adjusted model: Pain VAS adjusted for time varying CDAI, age, gender, race, health insurance type, college degree or above, work status, smoking history, BMI, history of comorbidities (infections, depression, anxiety, fibromyalgia, other comorbidities), number of comorbidities, duration of RA, modified HAQ, concomitant therapy, prior conventional DMARD use, prior bDMARD use, current opioid use, prednisone use, patient-reported fatigue, morning stiffness, problems with sleeping
Model 2.2 - Fully adjusted model: Pain VAS adjusted for time varying modified CDAI, age, gender, race, health insurance type, college degree or above, work status, smoking history, BMI, history of comorbidities (infections, depression, anxiety, fibromyalgia, other comorbidities), number of comorbidities, duration of RA, modified HAQ, concomitant therapy, prior cDMARD use, prior bDMARD use, current opioid use, prednisone use, patient-reported fatigue, morning stiffness, problems with sleeping
Model 3.1 - CDAI adjusted model: Pain VAS adjusted for time varying CDAI
Model 3.2 - mCDAI adjusted model: Pain VAS adjusted for time varying modified CDAI.
Disclosures: J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; J. Symons, GlaxoSmithKline (GSK); J. Janak, CorEvitas LLC; O. Pugach, CorEvitas LLC; E. Kohl, CorEvitas LLC; M. Yu, CorEvitas LLC; D. Webb, GlaxoSmithKline (GSK); A. Martin, GlaxoSmithKline (GSK); D. Saurigny, GlaxoSmithKline (GSK); M. Bracher, GlaxoSmithKline (GSK).
Background/Purpose: For many patients with RA on targeted therapies, the chronic burden of pain leads to therapies being deemed suboptimally effective and may affect treatment decisions. Our objective was to explore the association between patient-reported pain and switching biologic/targeted synthetic (b/ts)DMARDs.
Methods: Patients in the CorEvitas RA registry who met the following criteria were included: started a b/tsDMARD as of 1/1/2012; had ≥2 routine clinical visits; and no b/tsDMARD interruption (defined as being off treatment for >2 months). The outcome of interest was time to treatment switch to a b/tsDMARD with a different MOA. The exposure of interest was time-varying patient-reported pain measured on the visual analog scale (Pain VAS: 0–100 mm): mild (0–44 mm), moderate (45–74 mm), and severe (75–100 mm). Sociodemographics, lifestyle, disease characteristics, treatment history, and patient-reported outcomes were explored as potential confounders. Disease activity was analyzed with both time-varying Clinical Disease Activity Index (CDAI) and modified (m)CDAI (minus patient global assessment and tender joint count). Descriptive statistics stratified by baseline pain category were reported. Hazard ratios (HR) and 95% confidence intervals were reported for unadjusted and adjusted Cox hazards models with cluster-robust standard errors.
Results: Among 7806 patients, 9587 b/tsDMARD starts (Table, Panel B) were included for the following pain categories: mild (n=4126; 43%), moderate (n=2976; 31%), and severe (n=2430; 25%), missing (n=55; 1%). In patients with severe pain vs mild pain (Table, Panel A), a greater proportion were smokers (23% vs 15%); obese (52% vs 40%); and there was a higher prevalence of depression (43% vs 28%) and fibromyalgia (12% vs 3%). A higher proportion with severe pain vs mild pain reported ≥2 previous bDMARDs (50% vs 38%) and use of opioids (49% vs 18%), prednisone (39% vs 26%), and anti-depressants (33% vs 20%). The unadjusted risk of switching b/tsDMARD was ~2 times higher for severe vs mild pain (HR=1.98; 1.60–2.44; Figure). The fully adjusted risk for severe vs mild pain after accounting for confounders (HR=1.37; 1.02–1.86) was similar to the model that only included time-varying CDAI as a confounder (HR=1.35; 1.08–1.69). Compared with models that incorporated only time-varying CDAI, the risk of switching for severe pain was higher when adjusting instead for only time-varying mCDAI (HR=1.51; 1.22–1.87).
Conclusion: Patients that report greater pain are more likely to switch to a b/tsDMARD of a different MOA; an observation that remained after adjusting for confounders, including time-varying disease activity. Overall, these findings suggest that persistent pain is common in RA and influences treatment decisions, particularly a switch to a different MOA. Approaches that reduce chronic pain in this population may help reduce treatment cycling.
This GSK-funded study (214024) was sponsored by CorEvitas, LLC, with editorial support by Fishawack Indicia Ltd, funded by GSK.
.jpg)
 Table. Descriptive characteristics of sociodemographic factors, health behaviors and characteristics, disease, and treatment characteristics at baseline, by total sample and Pain VAS groups.
Table. Descriptive characteristics of sociodemographic factors, health behaviors and characteristics, disease, and treatment characteristics at baseline, by total sample and Pain VAS groups. a Insurance type is not mutually exclusive
b Based on the Centers for Disease Control and Prevention (CDC) cut-offs for normal/underweight (under 25); overweight (25.0–29.9); and obese (30.0 and above)
c Infections include Joint/bursa, cellulitis/skin, sinusitis, Candida, diverticulitis, sepsis, pneumonia, bronchitis, gastroenteritis, meningitis/encephalitis, urinary tract, upper respiratory, tuberculosis, and other. Infections resulting in hospitalization or administration of IV antibiotics indicates serious infection.
d Prior non-biologic systemic use count does not include the current non-biologic systemic therapy, if applicable
e Prior biologics count does not include subject’s current biologic, biosimilars are not counted
f No pain and mild pain (0–44 mm), moderate pain (45–74 mm), severe pain (75–100 mm)
g The EuroQoL-5 Dimension (EQ-5D) computes an index value using the individual mobility, self-care, usual activities, pain or discomfort, and anxiety or depression responses from the EQ-5D quality-of-life instrument.
h 47 patients (55 therapy starts) had missing baseline pain
 Figure. Forest plots of HR for switching b/tsDMARD with categorical Pain VAS (mild pain as a reference group) across different models.
Figure. Forest plots of HR for switching b/tsDMARD with categorical Pain VAS (mild pain as a reference group) across different models.Note: Unadjusted model: Pain VAS as only covariate, sample size=9587, number of events=980, mean follow-up time=22.9 months, median follow-up time=15 months
Model 1 - Unadjusted model: time-varying Pain VAS categories as only covariate, low Pain VAS group as a reference
Model 2.1 - Fully adjusted model: Pain VAS adjusted for time varying CDAI, age, gender, race, health insurance type, college degree or above, work status, smoking history, BMI, history of comorbidities (infections, depression, anxiety, fibromyalgia, other comorbidities), number of comorbidities, duration of RA, modified HAQ, concomitant therapy, prior conventional DMARD use, prior bDMARD use, current opioid use, prednisone use, patient-reported fatigue, morning stiffness, problems with sleeping
Model 2.2 - Fully adjusted model: Pain VAS adjusted for time varying modified CDAI, age, gender, race, health insurance type, college degree or above, work status, smoking history, BMI, history of comorbidities (infections, depression, anxiety, fibromyalgia, other comorbidities), number of comorbidities, duration of RA, modified HAQ, concomitant therapy, prior cDMARD use, prior bDMARD use, current opioid use, prednisone use, patient-reported fatigue, morning stiffness, problems with sleeping
Model 3.1 - CDAI adjusted model: Pain VAS adjusted for time varying CDAI
Model 3.2 - mCDAI adjusted model: Pain VAS adjusted for time varying modified CDAI.
Disclosures: J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; J. Symons, GlaxoSmithKline (GSK); J. Janak, CorEvitas LLC; O. Pugach, CorEvitas LLC; E. Kohl, CorEvitas LLC; M. Yu, CorEvitas LLC; D. Webb, GlaxoSmithKline (GSK); A. Martin, GlaxoSmithKline (GSK); D. Saurigny, GlaxoSmithKline (GSK); M. Bracher, GlaxoSmithKline (GSK).

