Back
Abstract Session
Crystal arthropathies
Session: Abstracts: Metabolic and Crystal Arthropathies – Basic and Clinical Science (1579–1584)
1584: Colchicine or Prednisone for the Treatment of Acute Calcium Pyrophosphate Deposition Arthritis: A Multicenter Randomized Controlled Trial
Sunday, November 13, 2022
4:15 PM – 4:25 PM Eastern Time
Location: Room 114 Nutter Theatre
- TP
Tristan Pascart, MD, PhD
Groupement Hospitalier de l'Institut Catholique de Lille
Lomme, France
Presenting Author(s)
Tristan Pascart1, Pierre Robinet2, Sebastien Ottaviani3, Remi Leroy4, Nicolas Segaud5, Aurore Pacaud2, Hélène Luraschi2, Thibault Rabin2, Agathe Grandjean2, Xavier Deplanque6, Pierre Maciejasz6, Fabien Visade2, Alexandre Mackowiak2, Nicolas Baclet6, Antoine Lefebvre2, Jean-François Budzik2, Thomas Bardin7, Pascal Richette8, Laurène Norberciak2, Vincent Ducoulombier2 and Eric Houvenagel1, 1Groupement Hospitalier de l'Institut Catholique de Lille, Lomme, France, 2Hôpital Saint-Philibert, Lomme, France, 3Hopital Bichat-Claude Bernard, Paris, France, 4Centre Hospitalier de Dunkerque, Dunkerque, France, 5Centre Hospitalier d'Armentières, Armentières, France, 6Hôpital Saint-Vincent-de-Paul, Lille, France, 7Hôpital Lariboisiere, Paris, France, 8Department of Rheumatology, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, Paris, France
Background/Purpose: Treatment of acute CPP arthritis mainly relies on expert opinion, as there are no trials that assessed the efficacy of anti-inflammatory drugs, despite the high frequency of the disease. The objective of this study (COLCHICORT; clinicaltrial.gov: NCT03128905) was to test the equivalence between colchicine and prednisone in patients with acute CPPD arthritis.
Methods: This is a multicenter open-label randomized trial enrolling patients above 65 years of age and eGFR >30ml/min/1.73m2, presenting with an acute CPPD arthritis (duration of symptoms less than 36h), as defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on X-Rays or ultrasound.
Participants were randomized to receive either colchicine 1.5mg (1 mg and then 0.5 mg one hour later) at baseline and then 1mg on day 1 or oral prednisone 30mg at baseline and day 1. Patients were to receive systematically 1g of acetaminophen and 50mg tramadol TID during the first 24 hours. Patients were followed-up for 6 days. The primary outcome was change in pain (visual analogic scale (VAS); 0-100 mm)) at 24h. Equivalence between groups was set at less than 13 mm on the pain VAS at 24 h in the per-protocol analysis. Secondary outcomes were response to treatment defined by pain VAS improvement > 50% from baseline and/or a pain VAS < 40/100 at day 1 and 2. Adverse events (AEs) were assessed. A sample size of 56 participants per arm was calculated to demonstrate equivalence set at less than 13 mm on the pain VAS change at 24h with 80% power, two-sided type 1 error of 0.05, 10% loss to follow-up (1).
Results: A total of 112 hospitalized patients were randomized (mean age 86.1 ± 7.1 years; sex ratio F/M 73.4%)): n = 48 in the colchicine group and n = 46 in the prednisone group (per-protocol population (8 drop-outs, 10 major deviations)). Overall, 26.3% of participants had diabetes mellitus, and mean eGFR was 63.2 ± 20.2 ml/min/1.73m2. Acute CPPD arthritis was crystal-proven for n = 37 (39.4%), and affected mainly the knee (n = 44, 47.3%), the wrist (n = 19, 20.4%) and the ankle (n = 13, 14.0%)(Table 1).
At 24 hours, change in pain VAS was -36.6 ±32.1 mm in the colchicine group and -37.7±19.4 in the prednisone group, respectively. The difference in pain change (VAS) at 24h between prednisone and colchicine was -1.17 mm [-11.88 ; 9.54] showing equivalence between the 2 drugs. (Figure 2).
Improvement in pain was similar between groups throughout the 6 days of follow-up (Figure 1). There were 64.6% of responders in the colchicine group and 66.7% in the prednisone group at 24h (p=1), and 63% and 72.7% in both groups (p=0.45) at 48h, respectively.
AEs were observed in n = 28 (50.9%) patients of the colchicine group and n = 20 (37%) in the prednisone group (p=0.21). The main AE in the colchicine group was diarrhea (n = 15, 26.3% vs n = 4, 7.3%) while it was onset of hypertension (n = 7, 12.7% vs n = 1, 1.8%), hyperglycemia (n = 3, 5.5% vs n = 0, 0%), and insomnia (n = 3, 5.5% vs n = 0, 0%) in the prednisone group, all resolving.
Conclusion: This randomized trial showed efficacy equivalence of low dose colchicine and oral prednisone in acute CPP arthritis.
Reference
1. Todd KH, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27:485-9.
.jpg) Figure 1. Evolution of daily VAS pain (0-100 mm) during the 6 days of follow-up.
Figure 1. Evolution of daily VAS pain (0-100 mm) during the 6 days of follow-up.
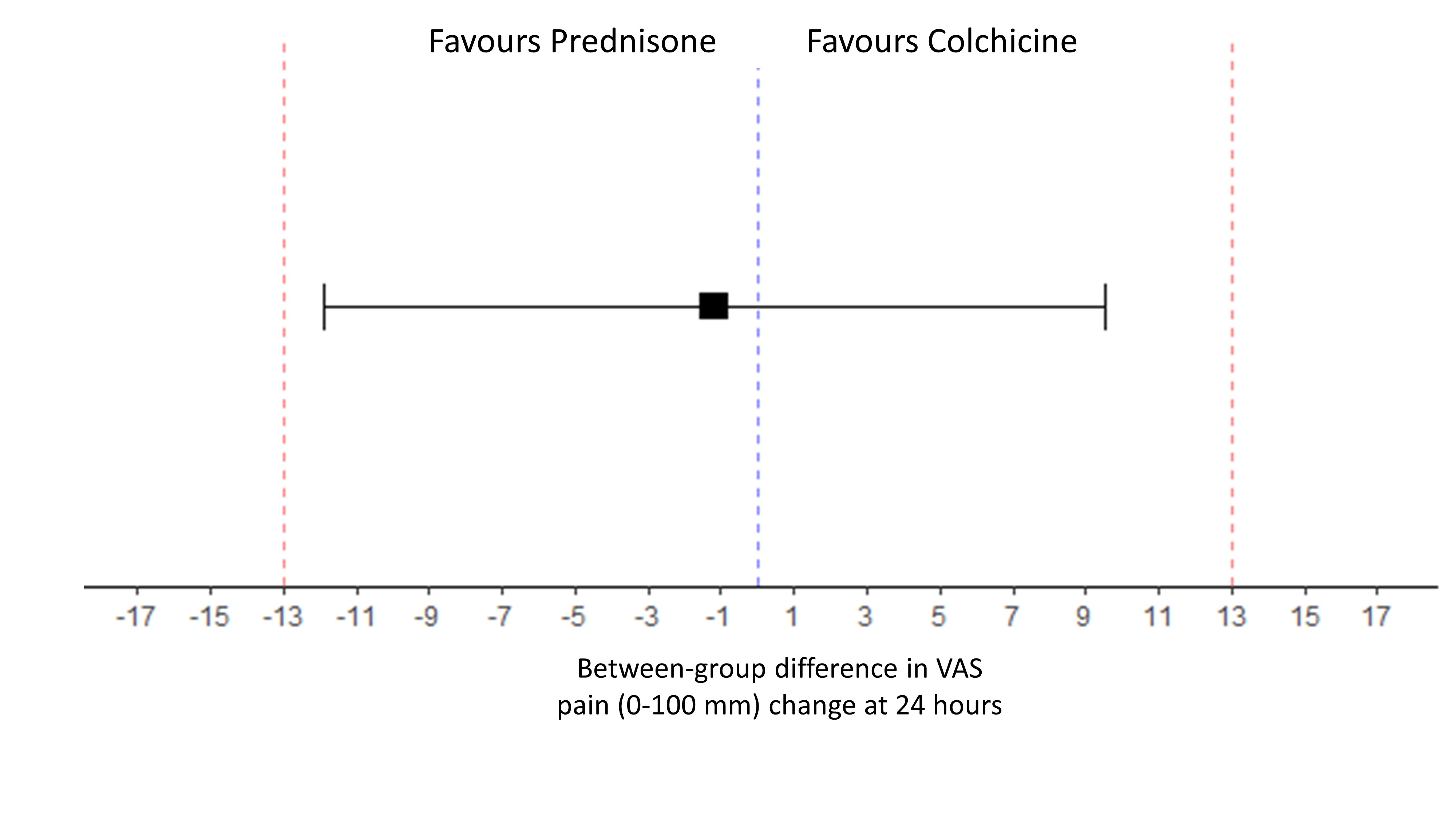 Figure 2. Forest plot for equivalence of Prednisone vs Colchicine on the evolution of VAS pain at 24 hours.
Figure 2. Forest plot for equivalence of Prednisone vs Colchicine on the evolution of VAS pain at 24 hours.
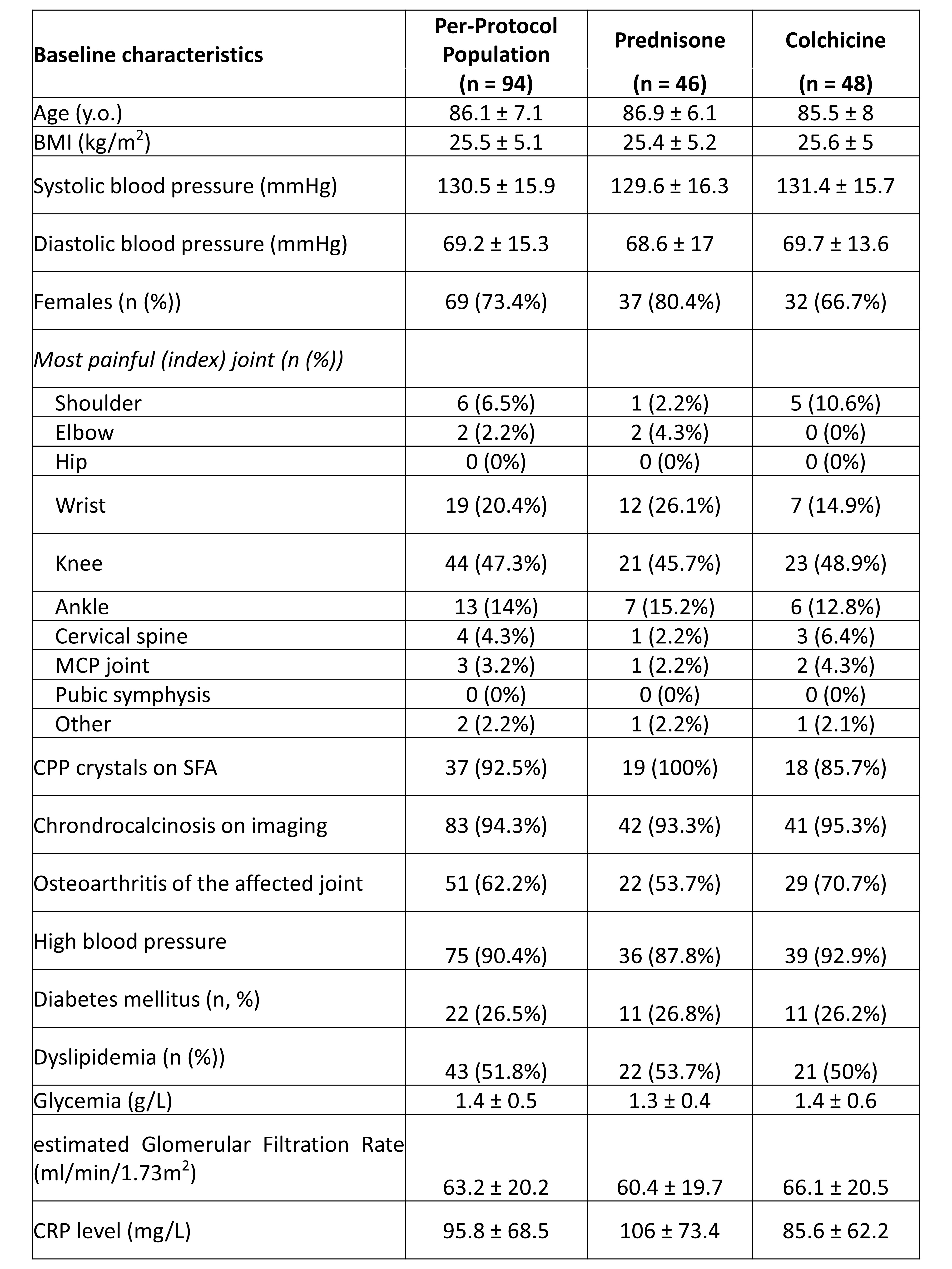 Table 1. Baseline patients’ characteristics.
Table 1. Baseline patients’ characteristics.
Disclosures: T. Pascart, None; P. Robinet, None; S. Ottaviani, None; R. Leroy, None; N. Segaud, None; A. Pacaud, None; H. Luraschi, None; T. Rabin, None; A. Grandjean, None; X. Deplanque, None; P. Maciejasz, None; F. Visade, None; A. Mackowiak, None; N. Baclet, None; A. Lefebvre, None; J. Budzik, None; T. Bardin, None; P. Richette, AbbVie, Amgen, Biogen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Sanofi-Aventis, UCB; L. Norberciak, None; V. Ducoulombier, None; E. Houvenagel, None.
Background/Purpose: Treatment of acute CPP arthritis mainly relies on expert opinion, as there are no trials that assessed the efficacy of anti-inflammatory drugs, despite the high frequency of the disease. The objective of this study (COLCHICORT; clinicaltrial.gov: NCT03128905) was to test the equivalence between colchicine and prednisone in patients with acute CPPD arthritis.
Methods: This is a multicenter open-label randomized trial enrolling patients above 65 years of age and eGFR >30ml/min/1.73m2, presenting with an acute CPPD arthritis (duration of symptoms less than 36h), as defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on X-Rays or ultrasound.
Participants were randomized to receive either colchicine 1.5mg (1 mg and then 0.5 mg one hour later) at baseline and then 1mg on day 1 or oral prednisone 30mg at baseline and day 1. Patients were to receive systematically 1g of acetaminophen and 50mg tramadol TID during the first 24 hours. Patients were followed-up for 6 days. The primary outcome was change in pain (visual analogic scale (VAS); 0-100 mm)) at 24h. Equivalence between groups was set at less than 13 mm on the pain VAS at 24 h in the per-protocol analysis. Secondary outcomes were response to treatment defined by pain VAS improvement > 50% from baseline and/or a pain VAS < 40/100 at day 1 and 2. Adverse events (AEs) were assessed. A sample size of 56 participants per arm was calculated to demonstrate equivalence set at less than 13 mm on the pain VAS change at 24h with 80% power, two-sided type 1 error of 0.05, 10% loss to follow-up (1).
Results: A total of 112 hospitalized patients were randomized (mean age 86.1 ± 7.1 years; sex ratio F/M 73.4%)): n = 48 in the colchicine group and n = 46 in the prednisone group (per-protocol population (8 drop-outs, 10 major deviations)). Overall, 26.3% of participants had diabetes mellitus, and mean eGFR was 63.2 ± 20.2 ml/min/1.73m2. Acute CPPD arthritis was crystal-proven for n = 37 (39.4%), and affected mainly the knee (n = 44, 47.3%), the wrist (n = 19, 20.4%) and the ankle (n = 13, 14.0%)(Table 1).
At 24 hours, change in pain VAS was -36.6 ±32.1 mm in the colchicine group and -37.7±19.4 in the prednisone group, respectively. The difference in pain change (VAS) at 24h between prednisone and colchicine was -1.17 mm [-11.88 ; 9.54] showing equivalence between the 2 drugs. (Figure 2).
Improvement in pain was similar between groups throughout the 6 days of follow-up (Figure 1). There were 64.6% of responders in the colchicine group and 66.7% in the prednisone group at 24h (p=1), and 63% and 72.7% in both groups (p=0.45) at 48h, respectively.
AEs were observed in n = 28 (50.9%) patients of the colchicine group and n = 20 (37%) in the prednisone group (p=0.21). The main AE in the colchicine group was diarrhea (n = 15, 26.3% vs n = 4, 7.3%) while it was onset of hypertension (n = 7, 12.7% vs n = 1, 1.8%), hyperglycemia (n = 3, 5.5% vs n = 0, 0%), and insomnia (n = 3, 5.5% vs n = 0, 0%) in the prednisone group, all resolving.
Conclusion: This randomized trial showed efficacy equivalence of low dose colchicine and oral prednisone in acute CPP arthritis.
Reference
1. Todd KH, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27:485-9.
.jpg) Figure 1. Evolution of daily VAS pain (0-100 mm) during the 6 days of follow-up.
Figure 1. Evolution of daily VAS pain (0-100 mm) during the 6 days of follow-up. Figure 2. Forest plot for equivalence of Prednisone vs Colchicine on the evolution of VAS pain at 24 hours.
Figure 2. Forest plot for equivalence of Prednisone vs Colchicine on the evolution of VAS pain at 24 hours. Table 1. Baseline patients’ characteristics.
Table 1. Baseline patients’ characteristics.Disclosures: T. Pascart, None; P. Robinet, None; S. Ottaviani, None; R. Leroy, None; N. Segaud, None; A. Pacaud, None; H. Luraschi, None; T. Rabin, None; A. Grandjean, None; X. Deplanque, None; P. Maciejasz, None; F. Visade, None; A. Mackowiak, None; N. Baclet, None; A. Lefebvre, None; J. Budzik, None; T. Bardin, None; P. Richette, AbbVie, Amgen, Biogen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Sanofi-Aventis, UCB; L. Norberciak, None; V. Ducoulombier, None; E. Houvenagel, None.

