Back
Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Diagnosis, Manifestations, and Outcomes II: Cardiovascular and Other Comorbidities (1646–1650)
1646: Major Adverse Cardiovascular Events and Mortality with Opioids versus NSAIDs Initiation in Patients with Rheumatoid Arthritis
Monday, November 14, 2022
9:00 AM – 9:10 AM Eastern Time
Location: Terrace Ballroom IV

Gulsen Ozen, MD
University of Nebraska Medical Center
Bellevue, NE, United States
Presenting Author(s)
Gulsen Ozen1, Sofia Pedro2 and Kaleb Michaud3, 1University of Nebraska Medical Center, Bellevue, NE, 2Forward, The National Databank for Rheumatic Diseases, Wichita, KS, 3University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Pain management is challenging in RA where ~60% of patients with well-controlled disease activity still experience bothersome pain. The opioid epidemic in the US has not spared patients with RA. Although adverse outcomes with NSAIDS for major cardiovascular events (MACE) have been shown, it is unknown if opioids are safe in RA where MACE risk is already increased. We aimed to assess MACE risk with opioids compared to NSAIDs in patients with RA.
Methods: We conducted a new-user active comparator cohort study within FORWARD, The National Databank for Rheumatic Diseases. The cohort included adult RA patients without cancer who participated ≥1 year in FORWARD between 1998 and 2021. Opioid initiators were matched with NSAID initiators by propensity scores (PS) that included age, sex, BMI, smoking, alcohol, RA duration, disease activity, HAQ, pain VAS, joint surgeries, prior cardiovascular disease (CVD) and venous thromboembolism (VTE), hypertension, diabetes, rheumatic diseases comorbidity index, osteoporosis/fractures, thyroid, chronic liver, kidney, lung and mental health diseases, hospitalizations, SF-36 and sleep scores, DMARDs, glucocorticoids, medications influencing MACE risk and calendar year. The subjects were followed up for the outcomes of MACE (myocardial infarction, stroke, heart failure, CVD death, VTE) and all-cause mortality. The risk of outcomes was estimated using Cox proportional hazards with adjustment for PS weights and imbalanced patient characteristics after matching. Patients were censored until the end of treatment + 3 months or end of follow-up if remained on treatment.
Results: The study included 4778 opioid and 11218 NSAID initiating RA patients. Baseline characteristics of opioid and NSAID initiators in the matched cohort were balanced except for medications for CVD including aspirin, antihypertensives, and nonstatin antilipidemics (Table 1). Among 4778 opioid initiators and 11218 NSAID initiators, 133 vs. 392 MACE (18.2/1000 person years [PY] vs. 14.6/1000 PY) and 95 vs. 228 deaths from any cause (12.6/1000 PY vs. 8.2/1000 PY) occurred, respectively (Table 2). Although incidence rates of MACE and all-cause mortality were lower in NSAID initiators than opioid initiators, the risk of MACE (HR=1.05, 95% CI 0.83-1.32) and all-cause mortality (HR=1.21, 95% CI 0.91-1.62) in PS weighted models were similar in opioid and NSAID initiators. Among the individual outcomes of MACE, VTE risk was significantly higher in opioid initiators than NSAID initiators (HR=2.45, 95% CI 1.27-4.74) (Figure).
Conclusion: Opioid initiation in RA has similar MACE and all-cause mortality risks compared to NSAID initiation except for almost 2-fold increased VTE risk. Our study, the first to assess comparative MACE/mortality risks of analgesics in RA with multiple relevant covariables, suggests that opioids may be not as safe as some clinicians have perceived with respect to MACE which is likely one of the main reasons for opioid selection over NSAIDs. Opioids with increased VTE risk and tendency of increased all-cause mortality risk in addition to known addiction/overdose potential and altered pain perception (hyperalgesia) may pose more risk than benefit in patients with RA.
.jpg) Table 1. Baseline characteristics of propensity score matched cohort of opioid and NSAID initiating RA patients
Table 1. Baseline characteristics of propensity score matched cohort of opioid and NSAID initiating RA patients
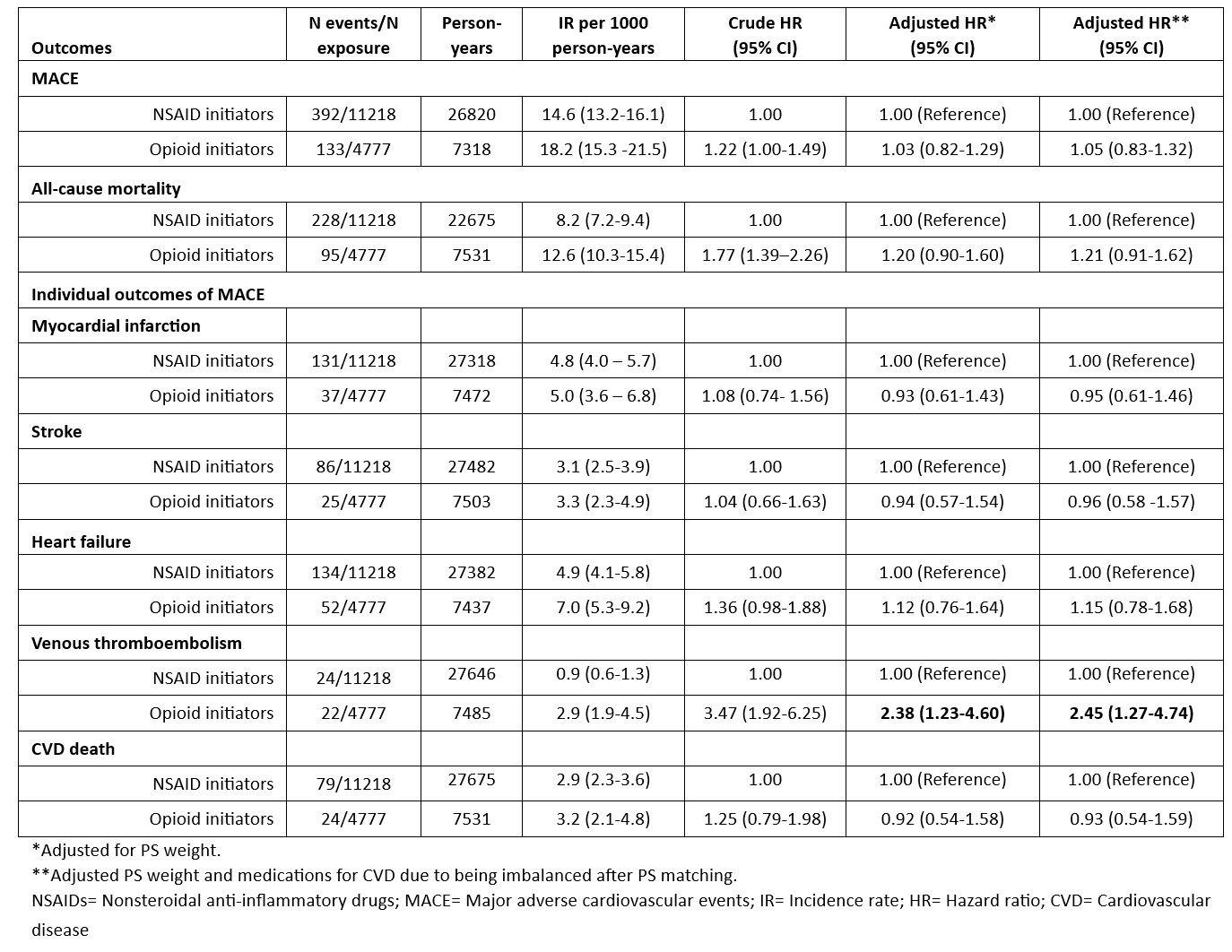 Table 2. Association of opioid initiation with MACE and all-cause mortality compared to NSAID initiation
Table 2. Association of opioid initiation with MACE and all-cause mortality compared to NSAID initiation
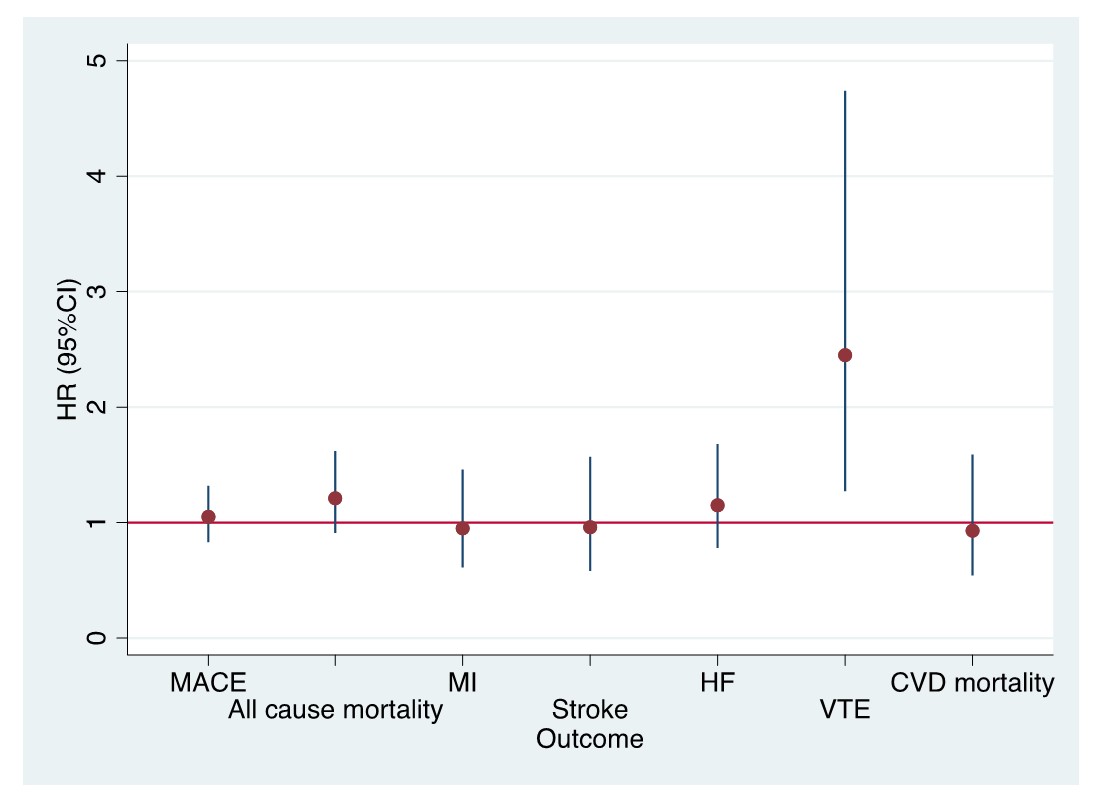 Figure. Risk of outcomes (HR, 95% CI) with opioid initiation compared to NSAID initiation after adjustment for propensity score weights and imbalanced covariables (CVD medications)
Figure. Risk of outcomes (HR, 95% CI) with opioid initiation compared to NSAID initiation after adjustment for propensity score weights and imbalanced covariables (CVD medications)
Disclosures: G. Ozen, None; S. Pedro, None; K. Michaud, None.
Background/Purpose: Pain management is challenging in RA where ~60% of patients with well-controlled disease activity still experience bothersome pain. The opioid epidemic in the US has not spared patients with RA. Although adverse outcomes with NSAIDS for major cardiovascular events (MACE) have been shown, it is unknown if opioids are safe in RA where MACE risk is already increased. We aimed to assess MACE risk with opioids compared to NSAIDs in patients with RA.
Methods: We conducted a new-user active comparator cohort study within FORWARD, The National Databank for Rheumatic Diseases. The cohort included adult RA patients without cancer who participated ≥1 year in FORWARD between 1998 and 2021. Opioid initiators were matched with NSAID initiators by propensity scores (PS) that included age, sex, BMI, smoking, alcohol, RA duration, disease activity, HAQ, pain VAS, joint surgeries, prior cardiovascular disease (CVD) and venous thromboembolism (VTE), hypertension, diabetes, rheumatic diseases comorbidity index, osteoporosis/fractures, thyroid, chronic liver, kidney, lung and mental health diseases, hospitalizations, SF-36 and sleep scores, DMARDs, glucocorticoids, medications influencing MACE risk and calendar year. The subjects were followed up for the outcomes of MACE (myocardial infarction, stroke, heart failure, CVD death, VTE) and all-cause mortality. The risk of outcomes was estimated using Cox proportional hazards with adjustment for PS weights and imbalanced patient characteristics after matching. Patients were censored until the end of treatment + 3 months or end of follow-up if remained on treatment.
Results: The study included 4778 opioid and 11218 NSAID initiating RA patients. Baseline characteristics of opioid and NSAID initiators in the matched cohort were balanced except for medications for CVD including aspirin, antihypertensives, and nonstatin antilipidemics (Table 1). Among 4778 opioid initiators and 11218 NSAID initiators, 133 vs. 392 MACE (18.2/1000 person years [PY] vs. 14.6/1000 PY) and 95 vs. 228 deaths from any cause (12.6/1000 PY vs. 8.2/1000 PY) occurred, respectively (Table 2). Although incidence rates of MACE and all-cause mortality were lower in NSAID initiators than opioid initiators, the risk of MACE (HR=1.05, 95% CI 0.83-1.32) and all-cause mortality (HR=1.21, 95% CI 0.91-1.62) in PS weighted models were similar in opioid and NSAID initiators. Among the individual outcomes of MACE, VTE risk was significantly higher in opioid initiators than NSAID initiators (HR=2.45, 95% CI 1.27-4.74) (Figure).
Conclusion: Opioid initiation in RA has similar MACE and all-cause mortality risks compared to NSAID initiation except for almost 2-fold increased VTE risk. Our study, the first to assess comparative MACE/mortality risks of analgesics in RA with multiple relevant covariables, suggests that opioids may be not as safe as some clinicians have perceived with respect to MACE which is likely one of the main reasons for opioid selection over NSAIDs. Opioids with increased VTE risk and tendency of increased all-cause mortality risk in addition to known addiction/overdose potential and altered pain perception (hyperalgesia) may pose more risk than benefit in patients with RA.
.jpg) Table 1. Baseline characteristics of propensity score matched cohort of opioid and NSAID initiating RA patients
Table 1. Baseline characteristics of propensity score matched cohort of opioid and NSAID initiating RA patients Table 2. Association of opioid initiation with MACE and all-cause mortality compared to NSAID initiation
Table 2. Association of opioid initiation with MACE and all-cause mortality compared to NSAID initiation Figure. Risk of outcomes (HR, 95% CI) with opioid initiation compared to NSAID initiation after adjustment for propensity score weights and imbalanced covariables (CVD medications)
Figure. Risk of outcomes (HR, 95% CI) with opioid initiation compared to NSAID initiation after adjustment for propensity score weights and imbalanced covariables (CVD medications)Disclosures: G. Ozen, None; S. Pedro, None; K. Michaud, None.

