Back
Abstract Session
Epidemiology, health policy and outcomes
Session: Abstracts: Epidemiology and Public Health I: Risk Factors and Outcomes (1633–1638)
1635: Safety Profile of Hydroxychloroquine in a Large Cohort of Rheumatology Patients Within the US Department of Defense Military Healthcare System
Monday, November 14, 2022
9:30 AM – 9:40 AM Eastern Time
Location: Room 204
- TR
Toni Rush, PhD, MPH
Health Research Tx
Pace, FL, United States
Presenting Author(s)
Toni Rush1, Rachel Robbins2, Angelique Collamer2 and Jess Edison3, 1Health Research Tx, Trevose, PA, 2Walter Reed National Military Medical Center, Bethesda, MD, 3Uniformed Services University of the Health Sciences / National Capital Consortium- Walter Reed Bethesda, Bethesda, MD
Background/Purpose: Hydroxychloroquine (HCQ) is a well-established conventional synthetic DMARD for many rheumatologic conditions. While generally believed to be safe, HCQ use is associated with a wide range of adverse effects with little knowledge of their true frequency. We set out to define the incidence and estimate the risk of known adverse events utilizing a large longitudinal database.
Methods: HCQ utilization was defined by retrospectively assessing pharmacy dispensing data for patients 18 years of age and older within the Department of Defense Military Health System between January 1, 2011 to December 31, 2019. Patients were grouped into the HCQ or non-HCQ cohort and assigned a rheumatology indication as stated below. All analyses were stratified by sex. Propensity score (PS) matching (1:1) was used to create study cohorts balanced on baseline covariates, including indication. Poisson regression modeling was used to estimate the relative risk of multiple outcomes after exposure to HCQ.
To control for confounding due to indication, a systematic algorithm was used to assign rheumatologic HCQ indication based on ICD-9/10 diagnostic codes assessed in claims data between January 1, 2010 to December 31, 2019. Patients with diagnostic codes for a single rheumatologic condition were assigned that condition for HCQ indication. If a patient had diagnostic codes for more than one rheumatologic condition, the indication with the most diagnostic codes was used as the defined HCQ indication. If one of the conditions was Sjögren's, then the condition other than Sjögren's' , e.g., rheumatoid arthritis, was assigned as the HCQ indication no matter the count of diagnostic codes. Systemic lupus erythematosus (SLE) took precedence when diagnostic codes for both SLE and discoid lupus were present.
Results: Within the study cohort, there were 44,117 female and 10,125 male patients with HCQ utilization. All HCQ patients were matched to non-HCQ patients to create both a female (n=88,234) and a male (n=20,250) matched cohort. The mean age of the matched female cohort was 55.8 years (sd 16.6) and male cohort was 60.8 years (sd 16.0). RA and SLE were the most prevalent indications for HCQ in both cohorts (Figure 1). The incidence of adverse outcomes varied within and between the cohorts. In both the female and male cohorts, HCQ exposure was associated with an increase risk in multiple outcomes with varying magnitudes by outcome and by sex (Table 2). One of the largest increase in risk was seen in toxic retinopathy for both the female and male patients (aRR 1.41, 95% CI 1.30, 1.48,p< .0001; aRR 1.39, 95% CI 1.25, 1.66, p< .0001; respectively) .Most commonly reported adverse events were found to occur in low, but statistically significant, rates (Table 2).
Conclusion: This uniquely large cohort of HCQ users reveals a relatively low incidence of previously described adverse events suggesting that the benefits of HCQ use outweigh the risks. In both men and women, the highest risks were for retinopathy and skin conditions highlighting the importance of regular ocular and dermatologic screening. Future studies will assess duration of HCQ use and timing of adverse events to further elucidate the safety profile of this vital treatment for rheumatology patients.
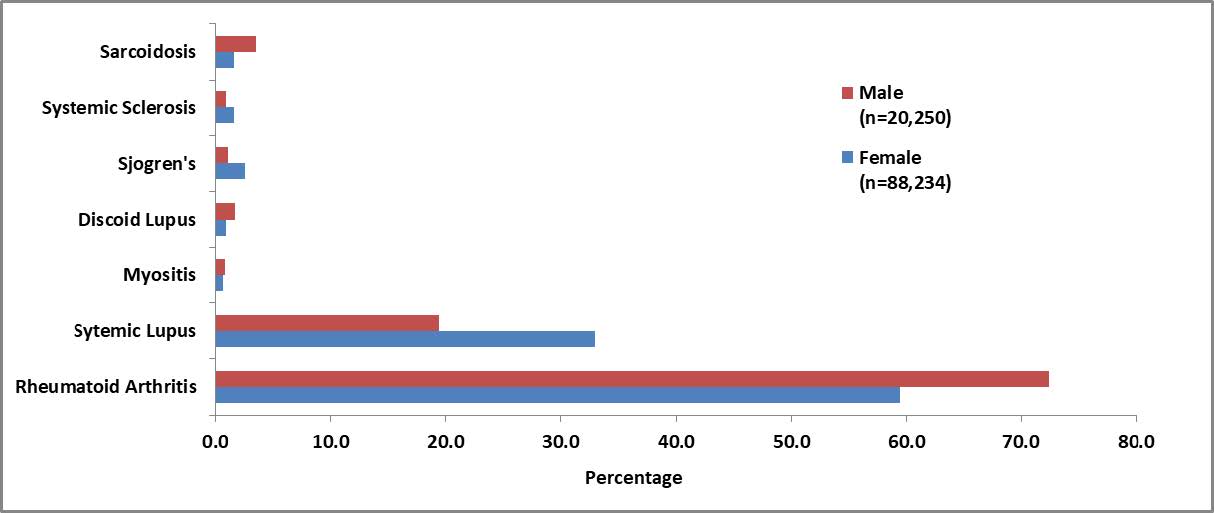 Figure 1. The proportions of rheumatology indications for HCQ represented in the PS-matched cohorts. Male patients represented in red (n=20,250) and Female patients represented in blue (n=88, 234).
Figure 1. The proportions of rheumatology indications for HCQ represented in the PS-matched cohorts. Male patients represented in red (n=20,250) and Female patients represented in blue (n=88, 234).
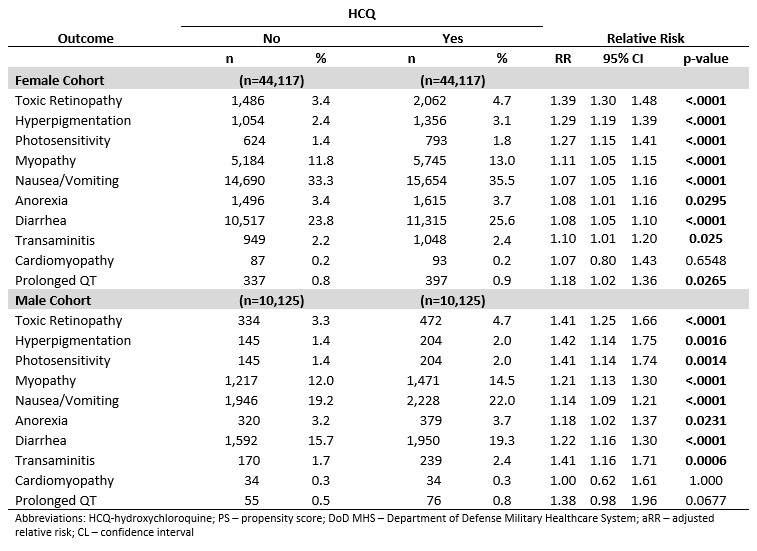 Table 1. Relative Risk of Outcomes Associated with HCQ Utilization among PS-matched, Adult Rheumatology Patients in the DoD MHS Database; Jan 01, 2011 − Dec 31, 2019
Table 1. Relative Risk of Outcomes Associated with HCQ Utilization among PS-matched, Adult Rheumatology Patients in the DoD MHS Database; Jan 01, 2011 − Dec 31, 2019

Disclosures: T. Rush, None; R. Robbins, None; A. Collamer, None; J. Edison, None.
Background/Purpose: Hydroxychloroquine (HCQ) is a well-established conventional synthetic DMARD for many rheumatologic conditions. While generally believed to be safe, HCQ use is associated with a wide range of adverse effects with little knowledge of their true frequency. We set out to define the incidence and estimate the risk of known adverse events utilizing a large longitudinal database.
Methods: HCQ utilization was defined by retrospectively assessing pharmacy dispensing data for patients 18 years of age and older within the Department of Defense Military Health System between January 1, 2011 to December 31, 2019. Patients were grouped into the HCQ or non-HCQ cohort and assigned a rheumatology indication as stated below. All analyses were stratified by sex. Propensity score (PS) matching (1:1) was used to create study cohorts balanced on baseline covariates, including indication. Poisson regression modeling was used to estimate the relative risk of multiple outcomes after exposure to HCQ.
To control for confounding due to indication, a systematic algorithm was used to assign rheumatologic HCQ indication based on ICD-9/10 diagnostic codes assessed in claims data between January 1, 2010 to December 31, 2019. Patients with diagnostic codes for a single rheumatologic condition were assigned that condition for HCQ indication. If a patient had diagnostic codes for more than one rheumatologic condition, the indication with the most diagnostic codes was used as the defined HCQ indication. If one of the conditions was Sjögren's, then the condition other than Sjögren's' , e.g., rheumatoid arthritis, was assigned as the HCQ indication no matter the count of diagnostic codes. Systemic lupus erythematosus (SLE) took precedence when diagnostic codes for both SLE and discoid lupus were present.
Results: Within the study cohort, there were 44,117 female and 10,125 male patients with HCQ utilization. All HCQ patients were matched to non-HCQ patients to create both a female (n=88,234) and a male (n=20,250) matched cohort. The mean age of the matched female cohort was 55.8 years (sd 16.6) and male cohort was 60.8 years (sd 16.0). RA and SLE were the most prevalent indications for HCQ in both cohorts (Figure 1). The incidence of adverse outcomes varied within and between the cohorts. In both the female and male cohorts, HCQ exposure was associated with an increase risk in multiple outcomes with varying magnitudes by outcome and by sex (Table 2). One of the largest increase in risk was seen in toxic retinopathy for both the female and male patients (aRR 1.41, 95% CI 1.30, 1.48,p< .0001; aRR 1.39, 95% CI 1.25, 1.66, p< .0001; respectively) .Most commonly reported adverse events were found to occur in low, but statistically significant, rates (Table 2).
Conclusion: This uniquely large cohort of HCQ users reveals a relatively low incidence of previously described adverse events suggesting that the benefits of HCQ use outweigh the risks. In both men and women, the highest risks were for retinopathy and skin conditions highlighting the importance of regular ocular and dermatologic screening. Future studies will assess duration of HCQ use and timing of adverse events to further elucidate the safety profile of this vital treatment for rheumatology patients.
 Figure 1. The proportions of rheumatology indications for HCQ represented in the PS-matched cohorts. Male patients represented in red (n=20,250) and Female patients represented in blue (n=88, 234).
Figure 1. The proportions of rheumatology indications for HCQ represented in the PS-matched cohorts. Male patients represented in red (n=20,250) and Female patients represented in blue (n=88, 234). Table 1. Relative Risk of Outcomes Associated with HCQ Utilization among PS-matched, Adult Rheumatology Patients in the DoD MHS Database; Jan 01, 2011 − Dec 31, 2019
Table 1. Relative Risk of Outcomes Associated with HCQ Utilization among PS-matched, Adult Rheumatology Patients in the DoD MHS Database; Jan 01, 2011 − Dec 31, 2019
Disclosures: T. Rush, None; R. Robbins, None; A. Collamer, None; J. Edison, None.

