Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
1648: Risk of Vascular Events Under the Treatments with Janus Kinase Inhibitors in Patients with Rheumatoid Arthritis: An Analysis Using Japanese Health Insurance Database
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- ET
Eiichi Tanaka, MD, PhD
Division of Rheumatology, Department of Internal Medicine, Tokyo Women’s Medical University School of Medicine
Tokyo, Japan
Abstract Poster Presenter(s)
Eiichi Tanaka1, Ryoko Sakai2, Eisuke Inoue3 and Masayoshi Harigai1, 1Division of Rheumatology, Department of Internal Medicine, Tokyo Women’s Medical University School of Medicine, Tokyo, Japan, 2Meiji Pharmaceutical University, Tokyo, Japan, 3Research Administration Center, Showa University, Shinagawa-ku, Japan
Background/Purpose: Janus kinase inhibitors (JAKIs) have shown positive therapeutic impacts on treatments for rheumatoid arthritis (RA), whereas, concerns have been raised about the risk of vascular events such as venous thromboembolism (VTE) based on the results from the clinical trials with a limited number of patients. Thus a population-based study in clinical settings is needed to investigate the safety of JAKIs in the real-world. The purpose of this study was to compare the risk of vascular events among JAKIs users, biological disease-modifying antirheumatic drugs (bDMARDs) (tumor necrosis factor inhibitors [TNFIs] and non-TNFIs) users, and methotrexate (MTX) users in patients with rheumatoid arthritis (RA) using a large health insurance claims data in Japan.
Methods: This retrospective longitudinal population-based study was conducted using claims data provided by Medical Data Vision Co., Ltd (Tokyo, Japan). We defined individuals as new users of JAKIs (tofacitinib, baricitinib, peficitinib, upadacitinib, filgotinib), bDMARDs or MTX if they met all of the following: 1) having at least one ICD10 code (M05 or M06); 2) having at least one prescription of JAKI, bDMARDs, or MTX between July 2013 and July 2020; 3) being over 18 years old; and 4) having prescription of neither JAKIs, bDMARDs nor MTX prior to six months before the index month. The index month was defined as the first month in which cases met all of the above criteria of 1), 2), and 3). Patients were followed from the index month until the last exposure to JAKIs, bDMARDs or MTX, date of loss of follow-up, or the end of follow-up (July 2021), whichever came first. Vascular events included VTE, arterial thrombosis, acute myocardial infarction, and stroke, and were defined when patients had at least one ICD code and medications for each disease. Patients were excluded from the study population if they had a vascular event during six months before the index month. We defined baseline characteristics using the data during the six months prior to the index month, and calculated incidence rate (IR), IR ratio (IRR, JAKIs vs. TNFIs, JAKIs vs. non-TNFI [IL6 inhibitors and abatacept [ABT]]), JAKIs vs. MTX), and adjusted hazard ratio (HR [95% CI]) of the vascular events using a time-dependent Cox regression model after adjusting for age at index month, sex, and comorbidities at baseline.
Results: In this study, 53,448 cases were included. The mean age was 64 years and 73% were female. Median observation period was 32 months. IRs/1,000 patient-years (PY) of the overall vascular events are shown in Table 1. Crude IRR of JAKIs vs. TNFIs was significantly increased, whereas IRRs of JAKIs vs. non-TNFIs as well as JAKIs vs. MTX were not. Adjusted HR of JAKIs for the overall vascular events was 1.7 [1.2–2.3] (vs. TNFIs), 1.4 [1.0–1.9] (vs. non-TNFIs), and 1.2 [0.9–1.6] (vs. MTX), respectively (Figure 1).
Conclusion: JAKIs user had a significantly higher risk of the overall vascular events than TNFIs and non-TNFIs users, and a similar risk to MTX user in patients with RA.
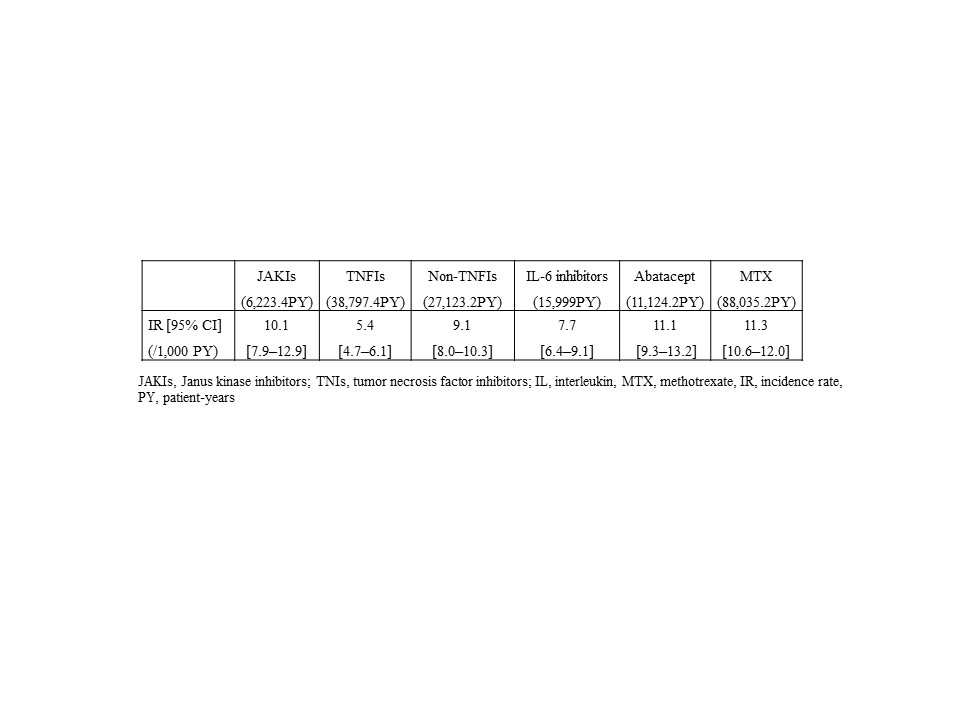 Table1 Crude incidence rate of the overall vascular events
Table1 Crude incidence rate of the overall vascular events
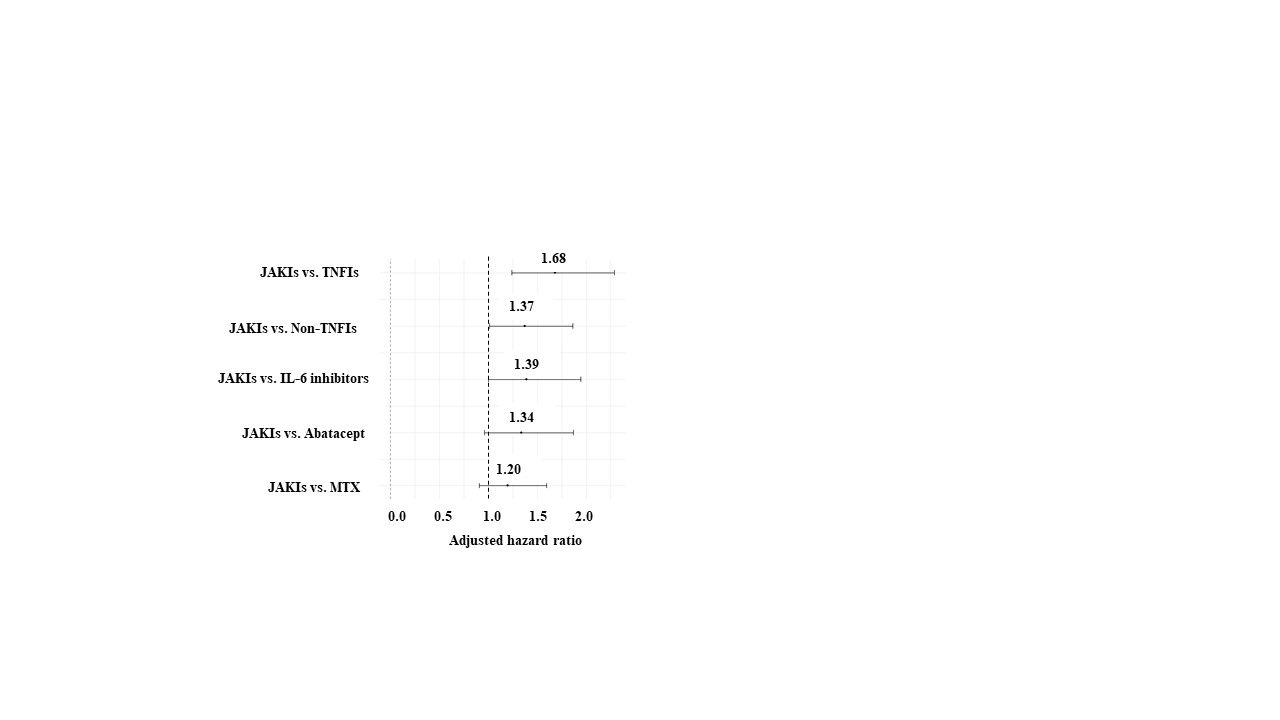 Figure 1 Adjusted hazard ratio of overall vascular events
Figure 1 Adjusted hazard ratio of overall vascular events
Disclosures: E. Tanaka, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Gilead Sciences, Inc., Kyowa Pharma Chemical CO., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Teijin Pharma Ltd., Takeda Pharmaceutical Co. , Ltd., UCB Japan Co. Ltd., Viatris Japan, AbbVie Japan GK, Pfizer Japan Inc., Janssen Pharmaceutical K.K.; R. Sakai, Chugai Pharmaceutical Co., Ltd., Nippon Kayaku, AYUMI Pharmaceutical Corporation, Asahi Kasei Pharma Corporation, Taisho Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd.; E. Inoue, Bristol-Myers Squibb(BMS), Nippontect systems; M. Harigai, AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., Viatris Japan.
Background/Purpose: Janus kinase inhibitors (JAKIs) have shown positive therapeutic impacts on treatments for rheumatoid arthritis (RA), whereas, concerns have been raised about the risk of vascular events such as venous thromboembolism (VTE) based on the results from the clinical trials with a limited number of patients. Thus a population-based study in clinical settings is needed to investigate the safety of JAKIs in the real-world. The purpose of this study was to compare the risk of vascular events among JAKIs users, biological disease-modifying antirheumatic drugs (bDMARDs) (tumor necrosis factor inhibitors [TNFIs] and non-TNFIs) users, and methotrexate (MTX) users in patients with rheumatoid arthritis (RA) using a large health insurance claims data in Japan.
Methods: This retrospective longitudinal population-based study was conducted using claims data provided by Medical Data Vision Co., Ltd (Tokyo, Japan). We defined individuals as new users of JAKIs (tofacitinib, baricitinib, peficitinib, upadacitinib, filgotinib), bDMARDs or MTX if they met all of the following: 1) having at least one ICD10 code (M05 or M06); 2) having at least one prescription of JAKI, bDMARDs, or MTX between July 2013 and July 2020; 3) being over 18 years old; and 4) having prescription of neither JAKIs, bDMARDs nor MTX prior to six months before the index month. The index month was defined as the first month in which cases met all of the above criteria of 1), 2), and 3). Patients were followed from the index month until the last exposure to JAKIs, bDMARDs or MTX, date of loss of follow-up, or the end of follow-up (July 2021), whichever came first. Vascular events included VTE, arterial thrombosis, acute myocardial infarction, and stroke, and were defined when patients had at least one ICD code and medications for each disease. Patients were excluded from the study population if they had a vascular event during six months before the index month. We defined baseline characteristics using the data during the six months prior to the index month, and calculated incidence rate (IR), IR ratio (IRR, JAKIs vs. TNFIs, JAKIs vs. non-TNFI [IL6 inhibitors and abatacept [ABT]]), JAKIs vs. MTX), and adjusted hazard ratio (HR [95% CI]) of the vascular events using a time-dependent Cox regression model after adjusting for age at index month, sex, and comorbidities at baseline.
Results: In this study, 53,448 cases were included. The mean age was 64 years and 73% were female. Median observation period was 32 months. IRs/1,000 patient-years (PY) of the overall vascular events are shown in Table 1. Crude IRR of JAKIs vs. TNFIs was significantly increased, whereas IRRs of JAKIs vs. non-TNFIs as well as JAKIs vs. MTX were not. Adjusted HR of JAKIs for the overall vascular events was 1.7 [1.2–2.3] (vs. TNFIs), 1.4 [1.0–1.9] (vs. non-TNFIs), and 1.2 [0.9–1.6] (vs. MTX), respectively (Figure 1).
Conclusion: JAKIs user had a significantly higher risk of the overall vascular events than TNFIs and non-TNFIs users, and a similar risk to MTX user in patients with RA.
 Table1 Crude incidence rate of the overall vascular events
Table1 Crude incidence rate of the overall vascular events Figure 1 Adjusted hazard ratio of overall vascular events
Figure 1 Adjusted hazard ratio of overall vascular eventsDisclosures: E. Tanaka, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Gilead Sciences, Inc., Kyowa Pharma Chemical CO., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Teijin Pharma Ltd., Takeda Pharmaceutical Co. , Ltd., UCB Japan Co. Ltd., Viatris Japan, AbbVie Japan GK, Pfizer Japan Inc., Janssen Pharmaceutical K.K.; R. Sakai, Chugai Pharmaceutical Co., Ltd., Nippon Kayaku, AYUMI Pharmaceutical Corporation, Asahi Kasei Pharma Corporation, Taisho Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd.; E. Inoue, Bristol-Myers Squibb(BMS), Nippontect systems; M. Harigai, AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., Viatris Japan.

