Back
Abstract Session
Epidemiology, health policy and outcomes
Session: Abstracts: Epidemiology and Public Health I: Risk Factors and Outcomes (1633–1638)
1637: Association of Dermatomyositis with Cardiovascular Disease: A Case-Control Study in the All of Us Research Program
Monday, November 14, 2022
10:00 AM – 10:10 AM Eastern Time
Location: Room 204
- JS
Jill Shah, BA
NYU Grossman School of Medicine
Albertson, NY, United States
Presenting Author(s)
Jill Shah1, Keya Shah2, Daniel Mazori1, Avrom Caplan1, Emily Hejazi1 and Alisa Femia1, 1Ronald O. Perelman Department of Dermatology, New York University Langone Health, New York, NY, 2Department of Medicine, NYU Langone Hospital - Long Island, Mineola, NY
Background/Purpose: Previous studies on the association of dermatomyositis (DM) with cardiovascular (CV) disease have used combined idiopathic inflammatory myositis cohorts, included only non-United States (US) cohorts, included only inpatients, or have not included matched controls. We aimed to describe the burden and timing of CV disease in a demographically and geographically diverse sample of inpatients and outpatients with DM in the US.
Methods: We performed a nested, matched, case-control analysis based on diagnostic coding in the All of Us Registered Tier Dataset v5 (Table 1). We used nearest neighbor propensity score matching to select for age-, sex-, race-, and ethnicity-matched controls for each DM case. We compared CV comorbidities and their dates of diagnosis between cases and controls using Pearson's chi-squared test or Fisher's exact test for categorical variables and the unpaired t-test for continuous variables. A multivariable conditional logistic regression model was built by including comorbidities with significance of P < 0.1 in univariable analysis, followed by backward elimination of comorbidities with a significance of P > 0.1 or with evidence of collinearity. A sensitivity analysis was performed that excluded DM cases with comorbid systemic lupus erythematosus (SLE), rheumatoid arthritis, psoriasis, or systemic sclerosis.
Results: Of the 214,206 All of Us participants with electronic health record data, we identified 248 DM cases and 992 controls (Table 2). The mean follow-up time for DM cases was 7.1 ± 4.8 years. Compared to controls, DM cases were significantly associated with 14 of 14 tested CV comorbidities in univariable analysis: atrial fibrillation (AF), cerebrovascular disease (CVD), chronic kidney disease (CKD), chronic obstructive pulmonary disease, coronary artery disease, deep vein thrombosis, heart failure, hyperlipidemia, hypertensive disorder (HTN), myocardial infarction, peripheral artery disease, pulmonary embolism, type 2 diabetes (T2D), and valvular heart disease (VHD). Aside from HTN, which was diagnosed on average 3.6 years earlier in the DM cohort, comorbidities were diagnosed at similar ages between cases and controls. In multivariable analysis, CKD, CVD, T2D, and VHD remained significantly associated with DM (Table 3). In the sensitivity analysis, 154 cases and 616 controls were identified. Univariable analysis results were similar except AF was not a significant association. In multivariable analysis, CKD, T2D, and VHD remained significantly associated; the odds ratio for CVD was 2.11 (p = 0.086).
Conclusion: This study found an association between DM and T2D, which has been previously reported. Unique to this study is the strong association of DM with CKD and with VHD, which remained significant in multiple multivariable models. Elevated risk of CV disease has been established in chronic inflammatory states such as SLE. This study shows a similar association between CV disease and DM. It is necessary to establish if treatment of DM decreases risk of CV disease, as is the case in the treatment of other rheumatologic diseases. Our study is limited by ascertainment of diagnoses using electronic health records and a lack of data on clinical features of DM.
.jpg) Table 1. All of Us Database Diagnosis Search Terms.
Table 1. All of Us Database Diagnosis Search Terms.
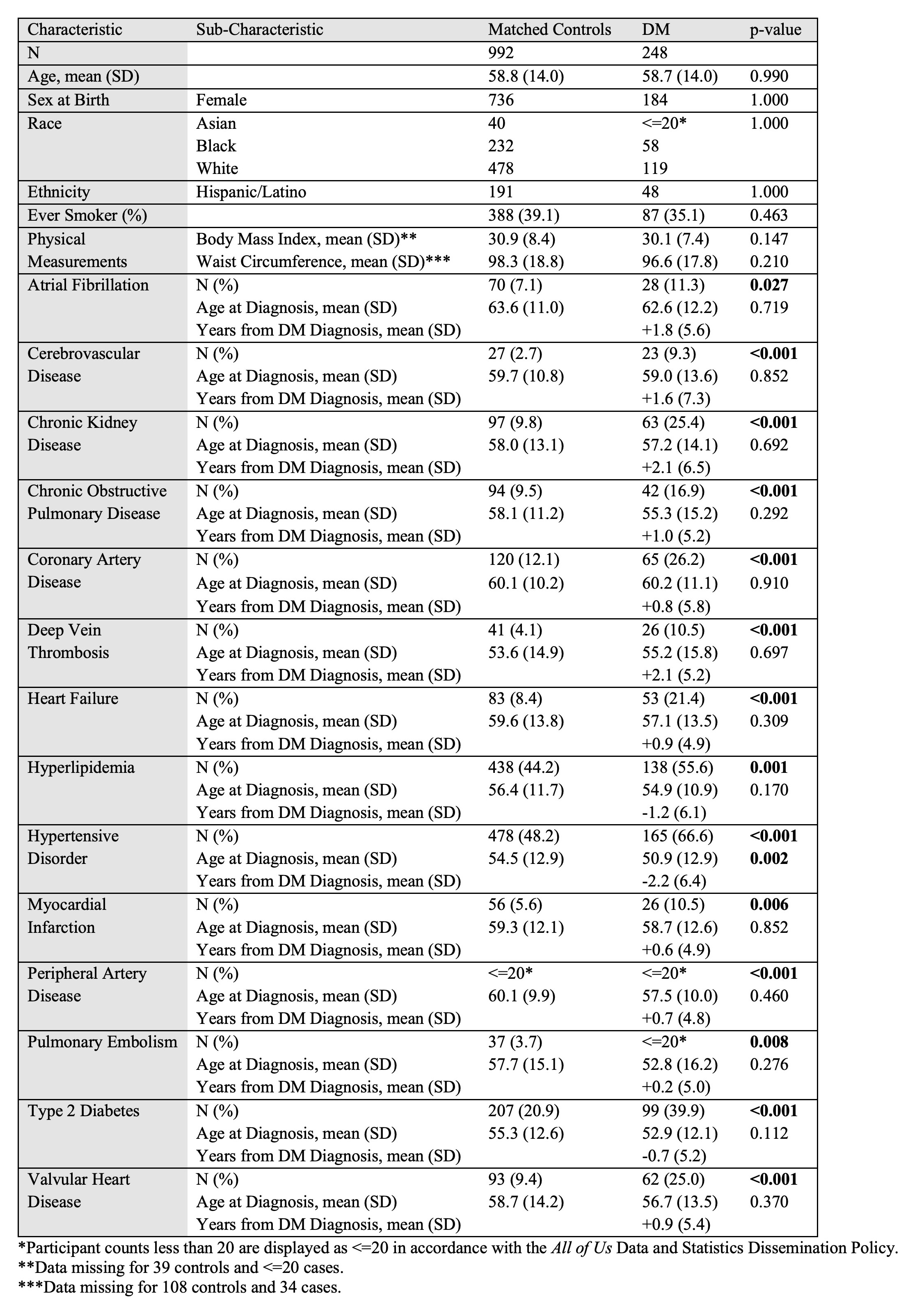 Table 2. Demographic and clinical characteristics of DM cases versus age-, sex-, race-, and ethnicity-matched controls in All of Us.
Table 2. Demographic and clinical characteristics of DM cases versus age-, sex-, race-, and ethnicity-matched controls in All of Us.
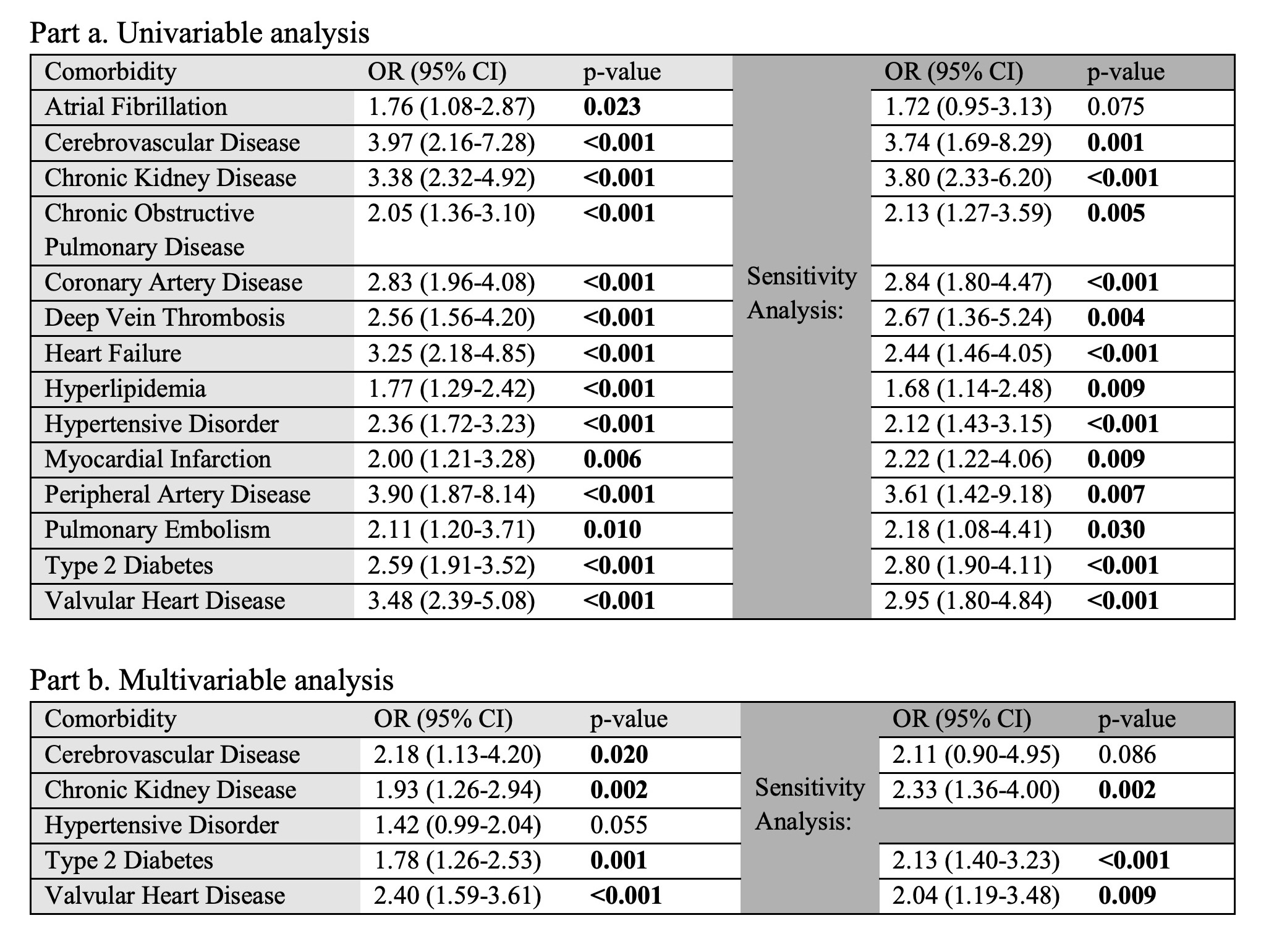 Table 3. Association of comorbidities with DM versus age-, sex-, race-, and ethnicity-matched controls as assessed by conditional logistic regression.
Table 3. Association of comorbidities with DM versus age-, sex-, race-, and ethnicity-matched controls as assessed by conditional logistic regression.
Disclosures: J. Shah, None; K. Shah, None; D. Mazori, None; A. Caplan, None; E. Hejazi, None; A. Femia, None.
Background/Purpose: Previous studies on the association of dermatomyositis (DM) with cardiovascular (CV) disease have used combined idiopathic inflammatory myositis cohorts, included only non-United States (US) cohorts, included only inpatients, or have not included matched controls. We aimed to describe the burden and timing of CV disease in a demographically and geographically diverse sample of inpatients and outpatients with DM in the US.
Methods: We performed a nested, matched, case-control analysis based on diagnostic coding in the All of Us Registered Tier Dataset v5 (Table 1). We used nearest neighbor propensity score matching to select for age-, sex-, race-, and ethnicity-matched controls for each DM case. We compared CV comorbidities and their dates of diagnosis between cases and controls using Pearson's chi-squared test or Fisher's exact test for categorical variables and the unpaired t-test for continuous variables. A multivariable conditional logistic regression model was built by including comorbidities with significance of P < 0.1 in univariable analysis, followed by backward elimination of comorbidities with a significance of P > 0.1 or with evidence of collinearity. A sensitivity analysis was performed that excluded DM cases with comorbid systemic lupus erythematosus (SLE), rheumatoid arthritis, psoriasis, or systemic sclerosis.
Results: Of the 214,206 All of Us participants with electronic health record data, we identified 248 DM cases and 992 controls (Table 2). The mean follow-up time for DM cases was 7.1 ± 4.8 years. Compared to controls, DM cases were significantly associated with 14 of 14 tested CV comorbidities in univariable analysis: atrial fibrillation (AF), cerebrovascular disease (CVD), chronic kidney disease (CKD), chronic obstructive pulmonary disease, coronary artery disease, deep vein thrombosis, heart failure, hyperlipidemia, hypertensive disorder (HTN), myocardial infarction, peripheral artery disease, pulmonary embolism, type 2 diabetes (T2D), and valvular heart disease (VHD). Aside from HTN, which was diagnosed on average 3.6 years earlier in the DM cohort, comorbidities were diagnosed at similar ages between cases and controls. In multivariable analysis, CKD, CVD, T2D, and VHD remained significantly associated with DM (Table 3). In the sensitivity analysis, 154 cases and 616 controls were identified. Univariable analysis results were similar except AF was not a significant association. In multivariable analysis, CKD, T2D, and VHD remained significantly associated; the odds ratio for CVD was 2.11 (p = 0.086).
Conclusion: This study found an association between DM and T2D, which has been previously reported. Unique to this study is the strong association of DM with CKD and with VHD, which remained significant in multiple multivariable models. Elevated risk of CV disease has been established in chronic inflammatory states such as SLE. This study shows a similar association between CV disease and DM. It is necessary to establish if treatment of DM decreases risk of CV disease, as is the case in the treatment of other rheumatologic diseases. Our study is limited by ascertainment of diagnoses using electronic health records and a lack of data on clinical features of DM.
.jpg) Table 1. All of Us Database Diagnosis Search Terms.
Table 1. All of Us Database Diagnosis Search Terms.  Table 2. Demographic and clinical characteristics of DM cases versus age-, sex-, race-, and ethnicity-matched controls in All of Us.
Table 2. Demographic and clinical characteristics of DM cases versus age-, sex-, race-, and ethnicity-matched controls in All of Us. Table 3. Association of comorbidities with DM versus age-, sex-, race-, and ethnicity-matched controls as assessed by conditional logistic regression.
Table 3. Association of comorbidities with DM versus age-, sex-, race-, and ethnicity-matched controls as assessed by conditional logistic regression.Disclosures: J. Shah, None; K. Shah, None; D. Mazori, None; A. Caplan, None; E. Hejazi, None; A. Femia, None.

