Back
Poster Session D
Crystal arthropathies
Session: (1787–1829) Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
1815: Consistent Colchicine Use Is Associated with Decreased Major Adverse Cardiovascular Events in Patients with Gout and Established Cardiovascular Disease
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GH
Gary Ho, MD
NYU Langone Health
Brooklyn, NY, United States
Abstract Poster Presenter(s)
Gary Ho1, Michael Toprover2, Daria Crittenden3, binita Shah4 and Michael Pillinger5, 1New York University Grossman School of Medicine, VA New York Harbor Health Care System, Brooklyn, NY, 2Division of Rheumatology, New York University Grossman School of Medicine and Rheumatology Section, New York Harbor Health Care System, New York Campus of the U.S. Department of Veterans Affairs, New York, NY, 3CymaBay Therapeutics, New York University Grossman School of Medicine, Newark, CA, 4New York University Grossman School of Medicine, VA New York Harbor Health Care System, New York, NY, 5NYU Grossman School of Medicine, New York, NY
Background/Purpose: Patients with gout are more likely than those without to have traditional risk factors for cardiovascular (CV) disease. Furthermore, the chronic, low-level inflammation experienced by gout patients between flares may further increase CV risk beyond traditional risk factors.
Colchicine has proven efficacy for the secondary prevention of major adverse cardiovascular events (MACE) in all-comers with coronary artery disease (CAD) and has retrospectively been associated with reduced MACE in the general gout population. The objective of this study is to evaluate the association between colchicine use and MACE in gout patients with established CAD.
Methods: This retrospective cohort study followed patients with gout and established CAD within the VA NY Harbor Healthcare System from 2000 to 2009 (preceding the 2012 ACR Gout Treatment Guidelines, which limited colchicine use to acute flares and/or prophylaxis), who did or did not use colchicine (minimum > 30 continuous days prescription). Criteria for the diagnosis of gout included: crystal proven gout; ≥ 6/12 of the 1977 American Rheumatology Association (ARA) Gout Classification Criteria; ≥ 4/12 ARA criteria and a primary care diagnosis of gout; or a rheumatologist diagnosis of gout. Pre-existing CAD was defined as: primary care or cardiologist documentation of CAD; positive cardiac stress test; ≥ 50% stenosis on coronary angiography; prior myocardial infarction (MI); or prior coronary revascularization. The primary outcome was first MACE event, defined as non-fatal MI, coronary artery bypass graft, non-fatal stroke, or all-cause mortality. Pre-specified subgroup analyses assessed associations by percentage of time on colchicine.
Results: Among 1,689 patients with gout, 355 had established CAD (239 colchicine users and 116 nonusers). Colchicine users had higher LDL-cholesterol levels, and a more frequent history of percutaneous coronary intervention and/or MI, potentially indicating more severe baseline coronary disease. On primary analysis, the odds of MACE were not decreased with colchicine use (OR 1.28; 95% CI [0.69-2.35]). However, due to widely varying degrees of colchicine use, colchicine users were next separated into quartiles by percentage of time on colchicine (Figure 1): Quartile 1 (11.4% ± 4.9%), Quartile 2 (31.3% ± 7.4%), Quartile 3 (56.6% ± 7.8%), and Quartile 4 (88.5% ± 9.9%). Patients in Quartile 4 demonstrated significantly lower odds of MACE compared to those in the lowest three quartiles (OR 0.35; 95% CI [0.13-0.93]), along with trends towards similar reductions in subcomponents (Figure 2). Colchicine users also had numerically fewer MACE events during periods of active use compared with periods of lapse (not shown). Kaplan-Meier analysis revealed a difference in cumulative MACE over time, favoring the highest colchicine-use quartile (plog-rank = 0.01) (Figure 3).
Conclusion: Among patients with gout and CAD receiving colchicine, those with the most consistent colchicine use ( > 70% of observation period on colchicine) had lower odds of MACE when compared to less consistent use. These data support the cardiovascular benefit of colchicine in high-risk populations and suggests the need for prospective trials.
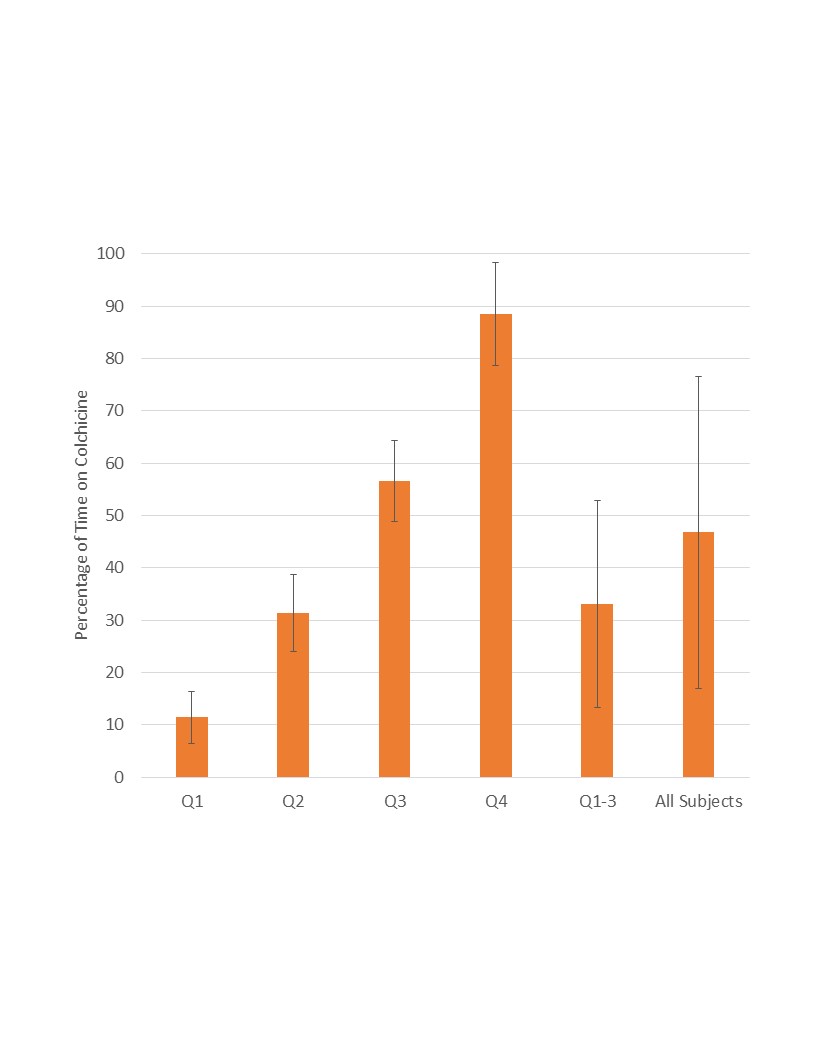 Mean and standard deviation percentage of time on colchicine within each quartile, Quartiles 1-3, and all subjects.
Mean and standard deviation percentage of time on colchicine within each quartile, Quartiles 1-3, and all subjects.
.jpg) Incidence of first MACE and sub-component events in Quartiles 1-3 versus Quartile 4.
Incidence of first MACE and sub-component events in Quartiles 1-3 versus Quartile 4.
.jpg) Kaplan-Meier survival curves in Quartiles 1-3 and Quartile 4 illustrating the cumulative proportion of patients experiencing a first MACE over time; maximum observation period of 10 years. Patients censored at the end of their respective observation periods are demarcated by a vertical line.
Kaplan-Meier survival curves in Quartiles 1-3 and Quartile 4 illustrating the cumulative proportion of patients experiencing a first MACE over time; maximum observation period of 10 years. Patients censored at the end of their respective observation periods are demarcated by a vertical line.
Disclosures: G. Ho, None; M. Toprover, Horizon Pharma; D. Crittenden, CymaBay Therapeutics; b. Shah, Horizons Therapeutic, Terumo medical, Philips Volcano; M. Pillinger, Horizon Therapeutics, Sobi, Fortress Bioscience, Hikma.
Background/Purpose: Patients with gout are more likely than those without to have traditional risk factors for cardiovascular (CV) disease. Furthermore, the chronic, low-level inflammation experienced by gout patients between flares may further increase CV risk beyond traditional risk factors.
Colchicine has proven efficacy for the secondary prevention of major adverse cardiovascular events (MACE) in all-comers with coronary artery disease (CAD) and has retrospectively been associated with reduced MACE in the general gout population. The objective of this study is to evaluate the association between colchicine use and MACE in gout patients with established CAD.
Methods: This retrospective cohort study followed patients with gout and established CAD within the VA NY Harbor Healthcare System from 2000 to 2009 (preceding the 2012 ACR Gout Treatment Guidelines, which limited colchicine use to acute flares and/or prophylaxis), who did or did not use colchicine (minimum > 30 continuous days prescription). Criteria for the diagnosis of gout included: crystal proven gout; ≥ 6/12 of the 1977 American Rheumatology Association (ARA) Gout Classification Criteria; ≥ 4/12 ARA criteria and a primary care diagnosis of gout; or a rheumatologist diagnosis of gout. Pre-existing CAD was defined as: primary care or cardiologist documentation of CAD; positive cardiac stress test; ≥ 50% stenosis on coronary angiography; prior myocardial infarction (MI); or prior coronary revascularization. The primary outcome was first MACE event, defined as non-fatal MI, coronary artery bypass graft, non-fatal stroke, or all-cause mortality. Pre-specified subgroup analyses assessed associations by percentage of time on colchicine.
Results: Among 1,689 patients with gout, 355 had established CAD (239 colchicine users and 116 nonusers). Colchicine users had higher LDL-cholesterol levels, and a more frequent history of percutaneous coronary intervention and/or MI, potentially indicating more severe baseline coronary disease. On primary analysis, the odds of MACE were not decreased with colchicine use (OR 1.28; 95% CI [0.69-2.35]). However, due to widely varying degrees of colchicine use, colchicine users were next separated into quartiles by percentage of time on colchicine (Figure 1): Quartile 1 (11.4% ± 4.9%), Quartile 2 (31.3% ± 7.4%), Quartile 3 (56.6% ± 7.8%), and Quartile 4 (88.5% ± 9.9%). Patients in Quartile 4 demonstrated significantly lower odds of MACE compared to those in the lowest three quartiles (OR 0.35; 95% CI [0.13-0.93]), along with trends towards similar reductions in subcomponents (Figure 2). Colchicine users also had numerically fewer MACE events during periods of active use compared with periods of lapse (not shown). Kaplan-Meier analysis revealed a difference in cumulative MACE over time, favoring the highest colchicine-use quartile (plog-rank = 0.01) (Figure 3).
Conclusion: Among patients with gout and CAD receiving colchicine, those with the most consistent colchicine use ( > 70% of observation period on colchicine) had lower odds of MACE when compared to less consistent use. These data support the cardiovascular benefit of colchicine in high-risk populations and suggests the need for prospective trials.
 Mean and standard deviation percentage of time on colchicine within each quartile, Quartiles 1-3, and all subjects.
Mean and standard deviation percentage of time on colchicine within each quartile, Quartiles 1-3, and all subjects..jpg) Incidence of first MACE and sub-component events in Quartiles 1-3 versus Quartile 4.
Incidence of first MACE and sub-component events in Quartiles 1-3 versus Quartile 4..jpg) Kaplan-Meier survival curves in Quartiles 1-3 and Quartile 4 illustrating the cumulative proportion of patients experiencing a first MACE over time; maximum observation period of 10 years. Patients censored at the end of their respective observation periods are demarcated by a vertical line.
Kaplan-Meier survival curves in Quartiles 1-3 and Quartile 4 illustrating the cumulative proportion of patients experiencing a first MACE over time; maximum observation period of 10 years. Patients censored at the end of their respective observation periods are demarcated by a vertical line.Disclosures: G. Ho, None; M. Toprover, Horizon Pharma; D. Crittenden, CymaBay Therapeutics; b. Shah, Horizons Therapeutic, Terumo medical, Philips Volcano; M. Pillinger, Horizon Therapeutics, Sobi, Fortress Bioscience, Hikma.

