Back
Poster Session D
Sjögren's syndrome
Session: (2017–2051) Sjögren's Syndrome – Basic and Clinical Science Poster
2022: Outcome of Pregnancy in Women with Primary Sjögren’s Syndrome Compared to the General Population: The French Multicenter Prospective GR2 Study
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GM
Gregoire Martin de Fremont, MD
Université Paris Cité
Paris, France
Abstract Poster Presenter(s)
Gregoire Martin de Fremont1, Nathalie Costedoat-Chalumeau2, Rakiba Belkhir3, Gaelle Guettrot-Imbert4, Nathalie Morel4, Gaetane Nocturne5, Anna Molto6, Tiphaine Goulenok7, Elisabeth Diot8, Estibaliz Lazaro9, Laurent Perard10, Nicole Ferreira11, Maelle Le Besnerais12, Nicolas Limal7, Nihal Martis13, Noémie Abisror14, Odile Debouverie15, Christophe Richez16, Vincent Sobanski17, Francois Maurier18, Gaetan Sauvetre12, Herve Levesque12, Marie Agnes Timsit19, Nathalie Tieule13, Pauline Orquevaux20, GEFA Collaborative group21, Matthieu Mahevas22, Celine Lartigau Roussin23, Elodie Chauvet24, Emilie Berthoux25, Francoise Sarrot Reynauld26, Loic Raffray27, Marion Couderc28, Nicolas Martin Silva29, Noemie Jourde Chiche30, Nicolas Belhomme31, Thierry Thomas32, Vincent Poindron33, Viviane Queyrel13, Juliette Delforge34, Camille Le Ray35, Emmanuelle Pannier35, Xavier Mariette36, Veronique Le Guern4 and Raphaèle Seror37, 1Rheumatology department, Kremlin Bicêtre hospital, APHP, Paris, France, 2Inserm DR Paris 5, Paris, France, 3Rheumatology departement, Bicêtre, Paris-Saclay university, Le Kremlin Bicêtre, France, 4Hôpital Cochin, Paris, France, 5APHP, Le Kremlin Bicêtre, France, 6Rheumatology Department, Cochin Hospital, APHP, Paris, France, 7APHP, Paris, France, 8CHU Tours, Tours, France, 9Bordeaux Hospital University, Bordeaux, France, 10CHU Lyon, Lyon, France, 11department of Internal Medecine, CHU de Tours, Tours, France, 12CHU Rouen, Rouen, France, 13CHU Nice, Nice, France, 14Hopital Saint Antoine, Paris, France, 15CHU Poitiers, Poitiers, France, 16Université de Bordeaux, Bordeaux, France, 17Université de Lille, Lille, France, 18Hôpitaux privés de Metz, Metz, France, 19CHU Brest, Brest, France, 20CHU Reims, Reims, France, 21Saint Joseph Hospital, Marseille, France, 22CHU Mondor, Creteil, France, 23CH La Réunion, CH la Réunion, France, 24CH Cabestany, Cabestany, France, 25CH Saint Joseph Saint Luc, Lyon, France, 26CHU Grenoble, CHU Grenoble, France, 27CH St Denis Reunion, Saint Denis Reunion, France, 28University Hospital, Clermont-Ferrand, France, 29CHU Caen, Caen, France, 30APHM, Marseille, France, 31CHU Rennes, Rennes, France, 32CHU Saint Etienne, Saint Etienne, France, 33Immunologie clinique et médecine interne, Hôpitaux universitaires de Strasbourg, Strasbourg, France, 34Jean Verfier, Bobigny, France, 35Port Royal, Paris, France, 36Paris-Saclay University, Rueil Malmaison, Ile-de-France, France, 37University Hospital Paris-Saclay, Le Kremlin Bicêtre, France
Background/Purpose: In the context of primary Sjögren's syndrome (pSS), only a few retrospective studies using heterogeneous methods have investigated the risk of adverse pregnancy outcomes (APO) with inconsistent results (APO frequency ranging from similar to the general population to ≥50% of pregnancies). Moreover, these studies rarely analyzed the impact of pregnancy on the course of pSS. We here aimed to describe the outcome of pregnancies in pSS and compare it to that of the general population.
Methods: The GR2 study is a French prospective cohort of pregnancies in women affected with auto-immune diseases involving 76 centers. The ENP is a French national perinatal survey on a sample of around 14.000 births, repeated every 5 years. We included GR2 pSS women fulfilling ACR/EULAR 2016 criteria with an ongoing pregnancy at 13 weeks of gestation. EULAR Sjögren's Syndrome Disease Activity Index and Patient Reported Index (ESSDAI and ESSPRI) were recorded at the first trimester of each pregnancy (baseline or bESSDAI), each trimester and at delivery, and a cumulative ESSDAI (cESSDAI) was calculated, defined as the sum of each domain maximum score during follow-up before pregnancy. A pSS flare was defined as an increase 3 points of the ESSDAI. APO were defined as the occurrence of any of the following events: unexplained intrauterine foetal death
3 points of the ESSDAI. APO were defined as the occurrence of any of the following events: unexplained intrauterine foetal death  13 weeks, neonatal death, placental insufficiency (intrauterine growth restriction, preeclampsia/eclampsia, HELLP syndrome, and/or placental abruption) leading to a premature delivery < 37 weeks or small-for-gestational-age birth weight. We compared the risk of APO occurring ≥ 22 weeks of gestation in pSS pregnancies to general population pregnancies from the 2016 ENP report, after matching (ratio 1:4) on age, parity and residential area. We performed a sensitivity analysis including congenital atrio-ventricular block (cAVB) in APO (APOblock).
13 weeks, neonatal death, placental insufficiency (intrauterine growth restriction, preeclampsia/eclampsia, HELLP syndrome, and/or placental abruption) leading to a premature delivery < 37 weeks or small-for-gestational-age birth weight. We compared the risk of APO occurring ≥ 22 weeks of gestation in pSS pregnancies to general population pregnancies from the 2016 ENP report, after matching (ratio 1:4) on age, parity and residential area. We performed a sensitivity analysis including congenital atrio-ventricular block (cAVB) in APO (APOblock).
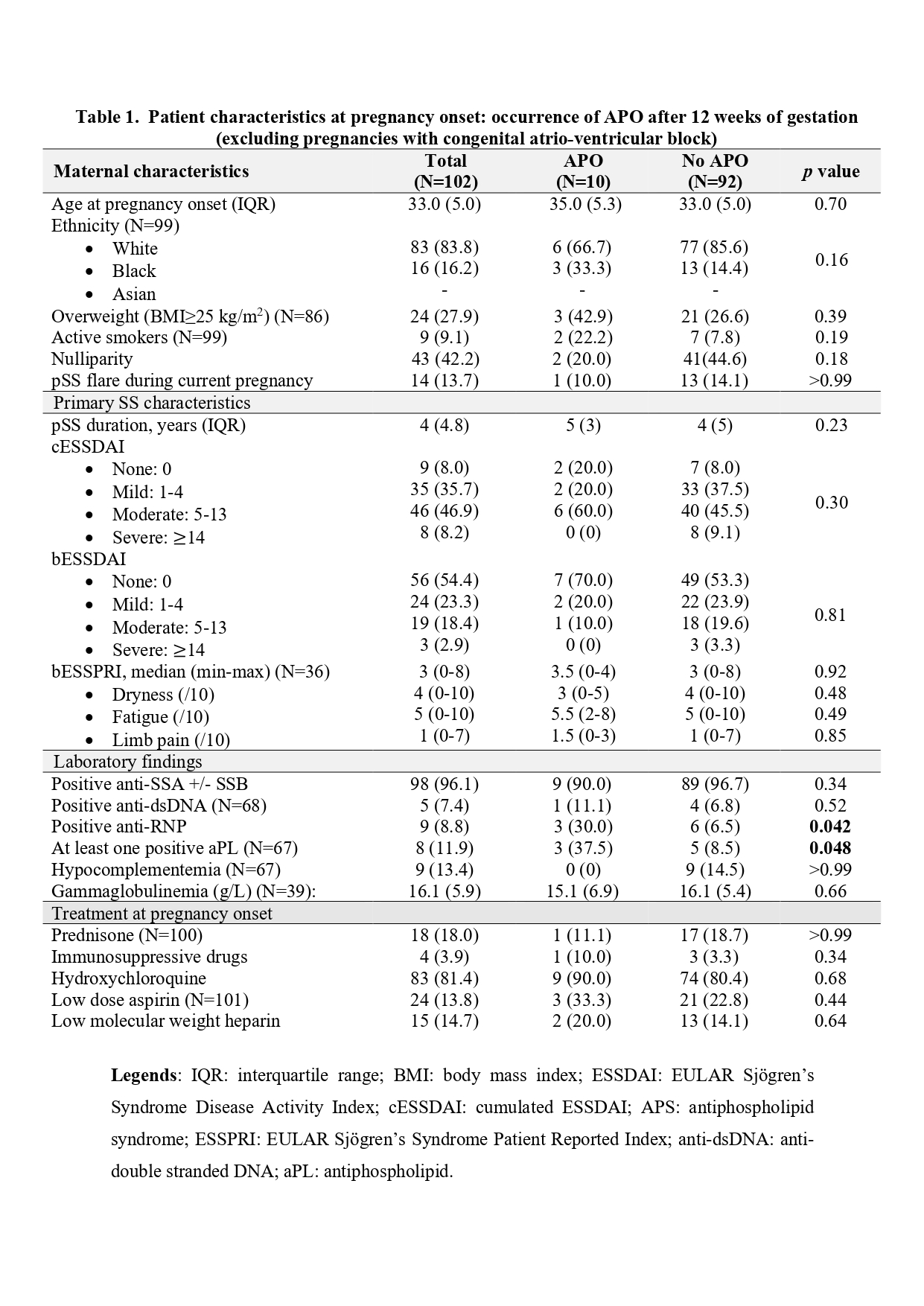
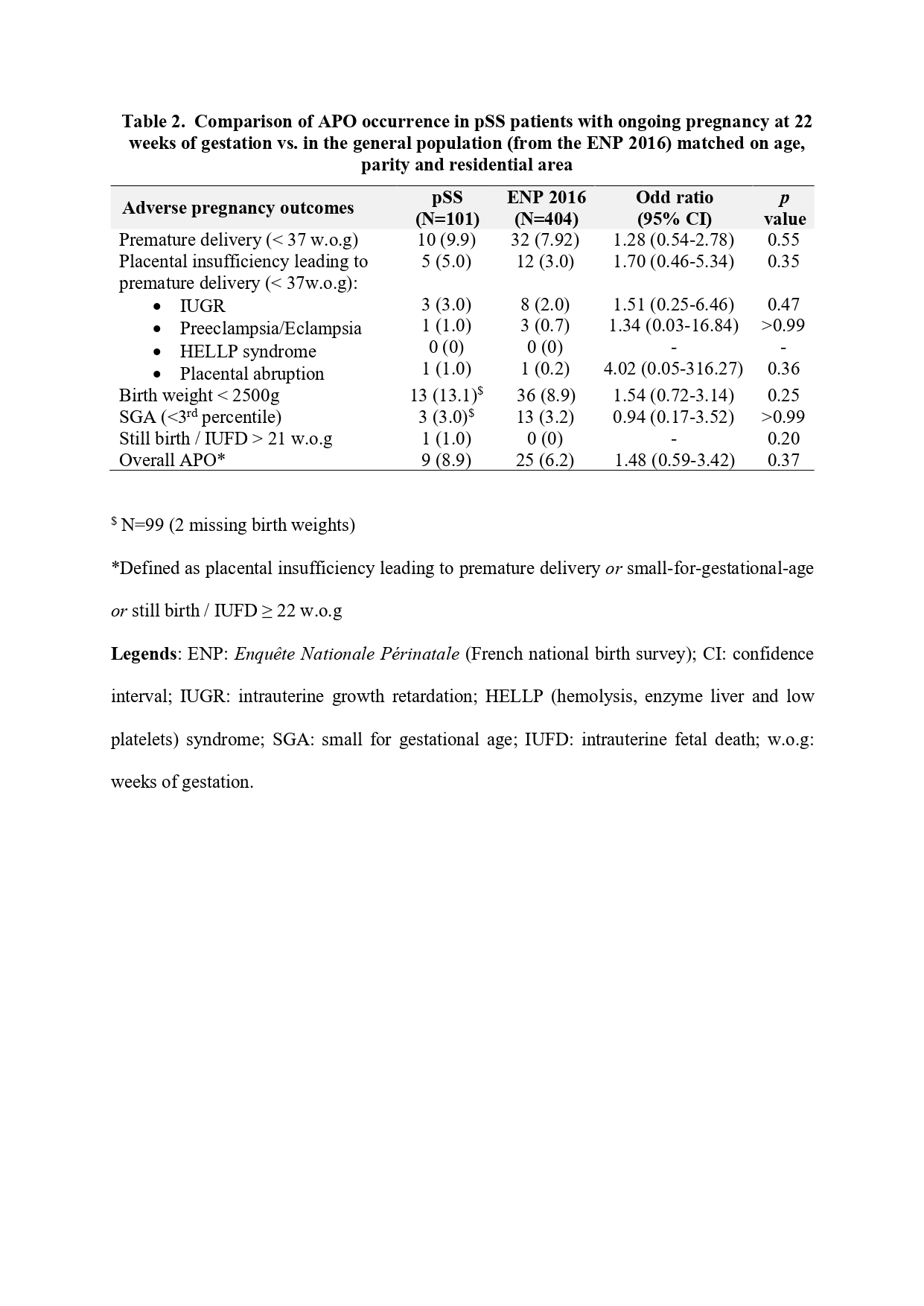
Results: 106 pregnancies occurred in 96 pSS women. pSS flares occurred in 14/106 (13.2%) pregnancies. Analyses did not identify any baseline parameter associated with the risk of pSS flare, in particular no association with ethnicity, cESSDAI, bESSDAI, or bESSPRI, biological markers of activity or type of autoantibodies. APO occurred in 10/102 (9.8%) [APOblock in 14/106 (13.2%)] pregnancies including only one with a pSS flare (Table 1). Women with and without APO had comparable age, weight, smoking status, cESSDAI, bESSDAI or bESSPRI at inclusion. However, women with APO had more often anti-RNP (30% vs 6.5%, p=0.042) and antiphospholipid (aPL) antibodies (n available=67, 37.5% vs 8.5%, p=0.048). Treatment exposure did not differ between groups. In sensitivity analysis, women with APOblock were more often Afro-Caribbean (38.5% vs 14.4%, p=0.049). APO frequency in pSS did not differ from that of the ENP population (8.9% vs 6.2%, OR 1.48 [0.59-3.42], p = 0.37) (Table 2).
Conclusion: APO and pSS flares were observed respectively in 9.8% and 13.2% of pregnancies but did not occur together. Anti-RNP and aPL antibodies were associated with a higher risk of APO, and ethnicity when including cAVB. APO frequency in pSS women was comparable to that of the general population.
Disclosures: G. Martin de Fremont, None; N. Costedoat-Chalumeau, UCB, Roche; R. Belkhir, None; G. Guettrot-Imbert, None; N. Morel, None; G. Nocturne, Pfizer, Novartis, Eli Lilly, Amgen; A. Molto, None; T. Goulenok, None; E. Diot, None; E. Lazaro, None; L. Perard, None; N. Ferreira, None; M. Le Besnerais, None; N. Limal, None; N. Martis, None; N. Abisror, None; O. Debouverie, None; C. Richez, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Galapados, GlaxoSmithKlein(GSK), Eli Lilly, Novartis, Pfizer; V. Sobanski, None; F. Maurier, None; G. Sauvetre, None; H. Levesque, None; M. Timsit, None; N. Tieule, None; P. Orquevaux, None; G. Collaborative group, None; M. Mahevas, None; C. Lartigau Roussin, None; E. Chauvet, None; E. Berthoux, None; F. Sarrot Reynauld, None; L. Raffray, None; M. Couderc, None; N. Martin Silva, None; N. Jourde Chiche, None; N. Belhomme, None; T. Thomas, None; V. Poindron, None; V. Queyrel, None; J. Delforge, None; C. Le Ray, None; E. Pannier, None; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; V. Le Guern, None; R. Seror, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Janssen, Novartis, Amgen.
Background/Purpose: In the context of primary Sjögren's syndrome (pSS), only a few retrospective studies using heterogeneous methods have investigated the risk of adverse pregnancy outcomes (APO) with inconsistent results (APO frequency ranging from similar to the general population to ≥50% of pregnancies). Moreover, these studies rarely analyzed the impact of pregnancy on the course of pSS. We here aimed to describe the outcome of pregnancies in pSS and compare it to that of the general population.
Methods: The GR2 study is a French prospective cohort of pregnancies in women affected with auto-immune diseases involving 76 centers. The ENP is a French national perinatal survey on a sample of around 14.000 births, repeated every 5 years. We included GR2 pSS women fulfilling ACR/EULAR 2016 criteria with an ongoing pregnancy at 13 weeks of gestation. EULAR Sjögren's Syndrome Disease Activity Index and Patient Reported Index (ESSDAI and ESSPRI) were recorded at the first trimester of each pregnancy (baseline or bESSDAI), each trimester and at delivery, and a cumulative ESSDAI (cESSDAI) was calculated, defined as the sum of each domain maximum score during follow-up before pregnancy. A pSS flare was defined as an increase
Results: 106 pregnancies occurred in 96 pSS women. pSS flares occurred in 14/106 (13.2%) pregnancies. Analyses did not identify any baseline parameter associated with the risk of pSS flare, in particular no association with ethnicity, cESSDAI, bESSDAI, or bESSPRI, biological markers of activity or type of autoantibodies. APO occurred in 10/102 (9.8%) [APOblock in 14/106 (13.2%)] pregnancies including only one with a pSS flare (Table 1). Women with and without APO had comparable age, weight, smoking status, cESSDAI, bESSDAI or bESSPRI at inclusion. However, women with APO had more often anti-RNP (30% vs 6.5%, p=0.042) and antiphospholipid (aPL) antibodies (n available=67, 37.5% vs 8.5%, p=0.048). Treatment exposure did not differ between groups. In sensitivity analysis, women with APOblock were more often Afro-Caribbean (38.5% vs 14.4%, p=0.049). APO frequency in pSS did not differ from that of the ENP population (8.9% vs 6.2%, OR 1.48 [0.59-3.42], p = 0.37) (Table 2).
Conclusion: APO and pSS flares were observed respectively in 9.8% and 13.2% of pregnancies but did not occur together. Anti-RNP and aPL antibodies were associated with a higher risk of APO, and ethnicity when including cAVB. APO frequency in pSS women was comparable to that of the general population.


Disclosures: G. Martin de Fremont, None; N. Costedoat-Chalumeau, UCB, Roche; R. Belkhir, None; G. Guettrot-Imbert, None; N. Morel, None; G. Nocturne, Pfizer, Novartis, Eli Lilly, Amgen; A. Molto, None; T. Goulenok, None; E. Diot, None; E. Lazaro, None; L. Perard, None; N. Ferreira, None; M. Le Besnerais, None; N. Limal, None; N. Martis, None; N. Abisror, None; O. Debouverie, None; C. Richez, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Galapados, GlaxoSmithKlein(GSK), Eli Lilly, Novartis, Pfizer; V. Sobanski, None; F. Maurier, None; G. Sauvetre, None; H. Levesque, None; M. Timsit, None; N. Tieule, None; P. Orquevaux, None; G. Collaborative group, None; M. Mahevas, None; C. Lartigau Roussin, None; E. Chauvet, None; E. Berthoux, None; F. Sarrot Reynauld, None; L. Raffray, None; M. Couderc, None; N. Martin Silva, None; N. Jourde Chiche, None; N. Belhomme, None; T. Thomas, None; V. Poindron, None; V. Queyrel, None; J. Delforge, None; C. Le Ray, None; E. Pannier, None; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; V. Le Guern, None; R. Seror, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Janssen, Novartis, Amgen.

