Back
Poster Session D
Osteoarthritis (OA) and related disorders
Session: (1888–1923) Osteoarthritis – Clinical Poster
1904: Is Treating Painful Hand Osteoarthritis with Hormone Replacement Therapy Feasible? Primary Results from a Randomised Controlled Trial in Post-menopausal Women
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- FW
Fiona Watt, PhD, MBBS, FRCP
Imperial College London
London, United Kingdom
Abstract Poster Presenter(s)
Jennifer Williams1, Mae Chester-Jones1, Megan Goff2, Anne Francis1, Gretchen Brewer1, Ioana Marian1, Malvika Gulati1, Charles Mackworth-Young3, Victoria Glover4, Sarah Lamb1, Tonia Vincent5, Katy Vincent1, Susan Dutton1 and Fiona Watt6, 1University of Oxford, Oxford, United Kingdom, 2University of Oxford, London, United Kingdom, 3Imperial College Healthcare NHS Trust, London, United Kingdom, 4White Horse Medical Practice, Faringdon, United Kingdom, 5Kennedy Institute of Rheumatology, University of Oxford, Oxford, United Kingdom, 6Imperial College London, London, United Kingdom
Background/Purpose: Hand osteoarthritis (OA) is more common in females and relative risk increases further around the age of menopause, potentially implicating sex hormone deficiency. There have been no randomised clinical trials (RCTs) of hormone replacement therapy (HRT) carried out in those with painful hand OA. We conducted a feasibility RCT of a form of HRT (conjugated estrogens (CE)-bazedoxifene) in post-menopausal women with painful hand OA.
Methods: Recruitment was at three primary/secondary care sites and from the community. Females, 40-65 yrs and 1-10 yrs after final menstrual period with definite hand OA and ≥2 painful hand joints were included. Study design was parallel group, double-blind 1:1 randomisation of CE-bazedoxifene or placebo, orally once daily for 24 weeks, then weaning and stopping by week 28. Primary feasibility outcomes were rates of eligible participant identification, recruitment, randomisation, retention, compliance, and likelihood of unblinding. Secondary outcomes were those proposed for a full trial (primary outcome: average hand pain over 14 days (by 2 methods, recall or daily average pain, rating 0-10); hand function (FIHOA), appearance, quality of life, menopause symptoms, satisfaction with medication and adverse events (AEs). Analysis was intention to treat. Treatment effects were difference in outcomes between the two arms, with 95% confidence intervals (study not powered to detect efficacy), with all models adjusted for hand OA subtype, study site and baseline values, where applicable. Progression criteria for a full RCT were (i) recruitment ≥30 participants in 18 months (ii) a drop out rate of ≤30% (iii) acceptability to the majority of participants, including AEs (ISRCTN12196200, funding NIHR, UK).
Results: Of 434 initial enquiries/referrals in 15 months (2019-2020), 250/401(62%) were ineligible and 55/401(14%) chose not to proceed. Of 96 telephone pre-screens, 28/35 proceeding (80%[63%,92%]) were eligible and randomised. Mean age was 58(SD3·4). High compliance and outcome measure completeness was recorded. Participants/investigators were well blinded (participant index 0·50(0·25,0·75)).
Average hand pain decreased from baseline to week 24 in both treatment arms (Fig1a&b). The methods showed good agreement and that there was no statistically significant evidence of a treatment effect (noting the study was not powered to test this). On weaning/stopping, average hand pain increased by mean 1·3 from week 24 to week 28 in the active arm compared with 0·17 on placebo (Fig1a). 46% and 17% in the active and placebo arms respectively reported worse pain at week 28 compared with week 24 (Fig1c). Predicted effects of HRT were detected for quality of life and menopause symptoms. The majority of participants were satisfied by the medication. All 3 AE-related treatment withdrawals were on placebo when unblinded. There were no serious AEs.
Conclusion: We report the first ever RCT of HRT for OA. The study met its 3 progression criteria. Though not powered to detect efficacy, upon medication withdrawal, an increase in average hand pain was seen in the active arm, indicating a possible effect. These data support a full trial of an HRT in this population being feasible, acceptable and worthwhile.
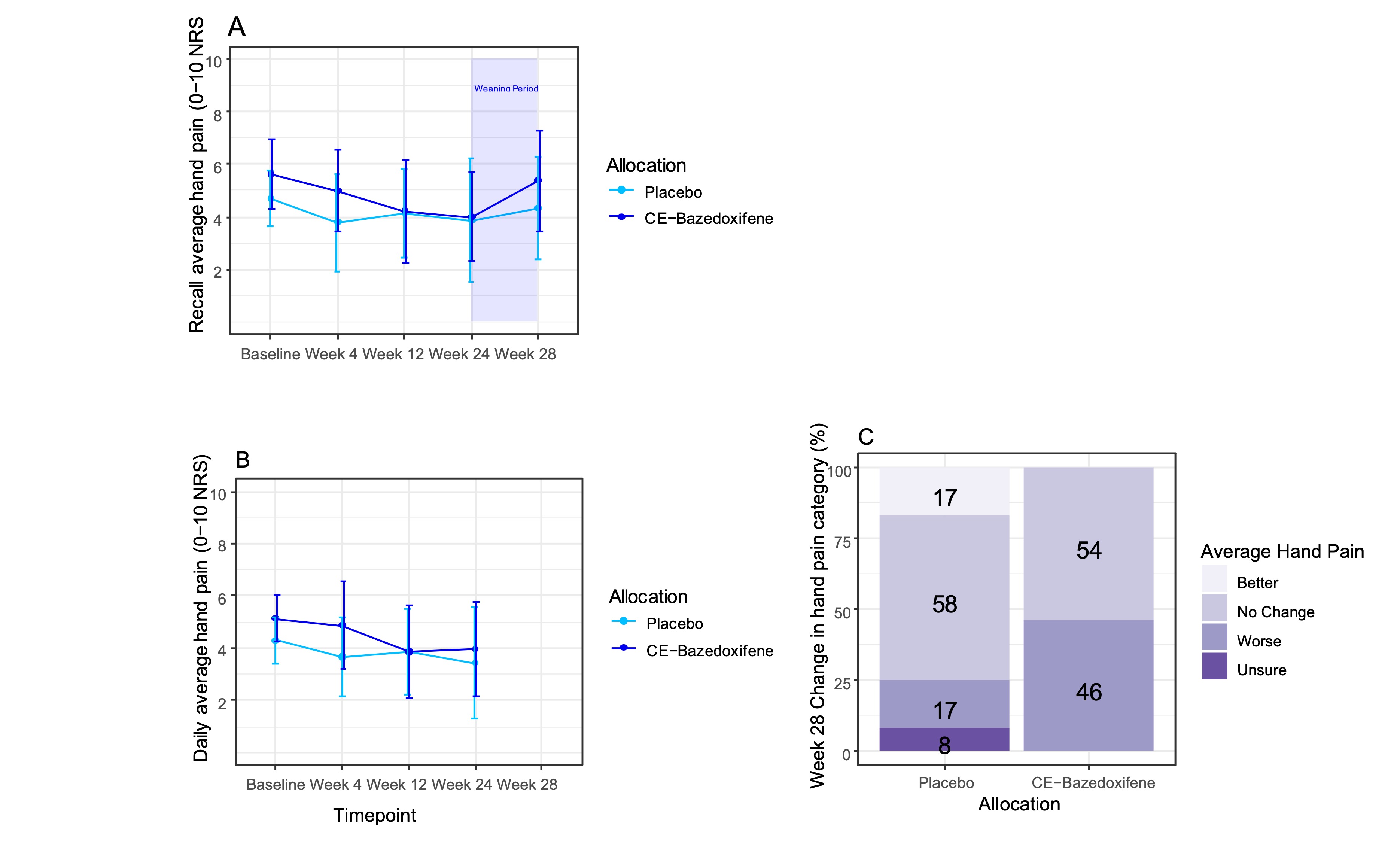 Figure 1
Figure 1
Disclosures: J. Williams, None; M. Chester-Jones, None; M. Goff, None; A. Francis, None; G. Brewer, None; I. Marian, None; M. Gulati, None; C. Mackworth-Young, None; V. Glover, None; S. Lamb, None; T. Vincent, Pfizer, Biosplice, Galapagos, Fidia, UCB, Novartis; K. Vincent, Grunenthal GmBH, Bayer Healthcare, AbbVie/Abbott, Eli Lilly; S. Dutton, None; F. Watt, Pfizer.
Background/Purpose: Hand osteoarthritis (OA) is more common in females and relative risk increases further around the age of menopause, potentially implicating sex hormone deficiency. There have been no randomised clinical trials (RCTs) of hormone replacement therapy (HRT) carried out in those with painful hand OA. We conducted a feasibility RCT of a form of HRT (conjugated estrogens (CE)-bazedoxifene) in post-menopausal women with painful hand OA.
Methods: Recruitment was at three primary/secondary care sites and from the community. Females, 40-65 yrs and 1-10 yrs after final menstrual period with definite hand OA and ≥2 painful hand joints were included. Study design was parallel group, double-blind 1:1 randomisation of CE-bazedoxifene or placebo, orally once daily for 24 weeks, then weaning and stopping by week 28. Primary feasibility outcomes were rates of eligible participant identification, recruitment, randomisation, retention, compliance, and likelihood of unblinding. Secondary outcomes were those proposed for a full trial (primary outcome: average hand pain over 14 days (by 2 methods, recall or daily average pain, rating 0-10); hand function (FIHOA), appearance, quality of life, menopause symptoms, satisfaction with medication and adverse events (AEs). Analysis was intention to treat. Treatment effects were difference in outcomes between the two arms, with 95% confidence intervals (study not powered to detect efficacy), with all models adjusted for hand OA subtype, study site and baseline values, where applicable. Progression criteria for a full RCT were (i) recruitment ≥30 participants in 18 months (ii) a drop out rate of ≤30% (iii) acceptability to the majority of participants, including AEs (ISRCTN12196200, funding NIHR, UK).
Results: Of 434 initial enquiries/referrals in 15 months (2019-2020), 250/401(62%) were ineligible and 55/401(14%) chose not to proceed. Of 96 telephone pre-screens, 28/35 proceeding (80%[63%,92%]) were eligible and randomised. Mean age was 58(SD3·4). High compliance and outcome measure completeness was recorded. Participants/investigators were well blinded (participant index 0·50(0·25,0·75)).
Average hand pain decreased from baseline to week 24 in both treatment arms (Fig1a&b). The methods showed good agreement and that there was no statistically significant evidence of a treatment effect (noting the study was not powered to test this). On weaning/stopping, average hand pain increased by mean 1·3 from week 24 to week 28 in the active arm compared with 0·17 on placebo (Fig1a). 46% and 17% in the active and placebo arms respectively reported worse pain at week 28 compared with week 24 (Fig1c). Predicted effects of HRT were detected for quality of life and menopause symptoms. The majority of participants were satisfied by the medication. All 3 AE-related treatment withdrawals were on placebo when unblinded. There were no serious AEs.
Conclusion: We report the first ever RCT of HRT for OA. The study met its 3 progression criteria. Though not powered to detect efficacy, upon medication withdrawal, an increase in average hand pain was seen in the active arm, indicating a possible effect. These data support a full trial of an HRT in this population being feasible, acceptable and worthwhile.
 Figure 1
Figure 1Disclosures: J. Williams, None; M. Chester-Jones, None; M. Goff, None; A. Francis, None; G. Brewer, None; I. Marian, None; M. Gulati, None; C. Mackworth-Young, None; V. Glover, None; S. Lamb, None; T. Vincent, Pfizer, Biosplice, Galapagos, Fidia, UCB, Novartis; K. Vincent, Grunenthal GmBH, Bayer Healthcare, AbbVie/Abbott, Eli Lilly; S. Dutton, None; F. Watt, Pfizer.

