Back
Poster Session D
Crystal arthropathies
Session: (1787–1829) Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
1806: Characteristics and Comorbidity Burden of Phase 3 Clinical Trial Participants Who Did and Did Not Experience Acute Gout Flares During Biweekly Pegloticase Dosing
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NS
Naomi Schlesinger, MD
Rutgers Robert Wood Johnson Medical School
New Brunswick, NJ, United States
Abstract Poster Presenter(s)
Naomi Schlesinger1, Lissa Padnick-Silver2, Katie Obermeyer2 and Brian LaMoreaux2, 1Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, 2Horizon Therapeutics plc, Deerfield, IL
Background/Purpose: Acute gout flare often accompanies urate-lowering therapy initiation and is the most common adverse event associated with pegloticase, a recombinant pegylated uricase that rapidly lowers serum uric acid (sUA).1 A recent case series of uncontrolled gout patients undergoing pegloticase plus methotrexate co-therapy found that patients who experienced acute gout flare during therapy were slightly older, more often obese, and had higher comorbidity burden at baseline than those that did not.2 To better understand these findings, this analysis examined and compared phase 3 pegloticase trial participants with and without gout flare during treatment.
Methods: Patients with uncontrolled gout, sUA≥8 mg/dL, and allopurinol intolerance/recalcitrance who were administered biweekly 8 mg pegloticase infusions as part of the pegloticase phase 3 pivotal trials1 were included. Patient baseline characteristics were compared in this post hoc analysis between participants who did and did not experience ≥1 acute gout flare during treatment.
Results: 66 patients (80.3% male, 57.0±15.12 years old, BMI: 33.0±7.42 kg/m2; sUA: 10.2±1.81 mg/dL) with ≥1 acute gout flare and 18 patients (77.8% males, 53.2±17.40 years old, BMI: 32.8±7.37 kg/m2; sUA=9.7±2.71 mg/dL) without acute gout flare were included. Patients who experienced ≥1 flare following pegloticase initiation had more acute gout flares in the 18 months prior to treatment (11.1±11.49 vs. 5.4±3.31 flares, p=0.040). Though many characteristics were similar, a larger proportion of flare patients were white (69.7% vs. 44.4%, p=0.057). 73.9% (207/280) of listed comorbidities were more prevalent in patients with ≥1 acute gout flare, indicating higher overall comorbidity burden; most notably, diabetes mellitus (30.3% vs. 16.7%; non-insulin dependent: 16.7% vs. 0%), renal failure (25.8% vs. 16.7%), and nervous system disorders (40.9% vs. 16.7%), but all p >0.05 (Figure). Additionally, more patients who experienced acute gout flares during treatment had insomnia (18.2% vs. 0) and depression (15.2% vs. 0) prior to treatment.
Conclusion: Findings of this post hoc analysis largely agree with those of a recent retrospective chart review study2 suggesting higher comorbidity in patients who experience acute gout flares during pegloticase therapy. We also found possible differences in patient race and pre-treatment flare rate. This small study is hypothesis generating and further study is needed on a larger population. However, identifying possible risk factors for acute gout flare following ULT initiation would be beneficial for both patient and physician.
References:
1. Sundy JS, et al. JAMA 2011, 306:711-20.
2. Albert J, et al. Ann Rheum Dis 2022:81(Suppl 1):1644.
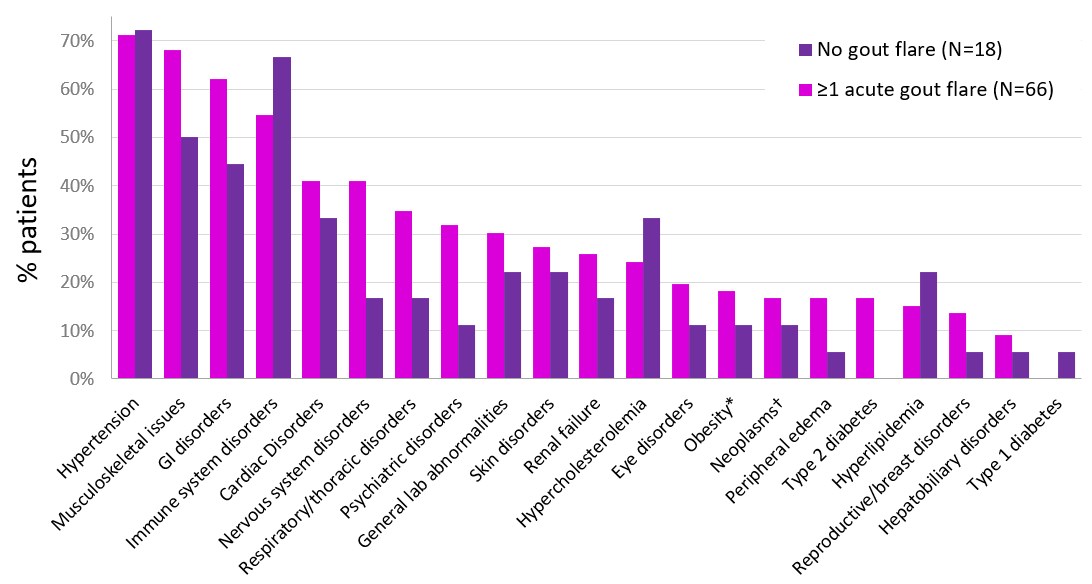 Figure. Comorbidities in phase 3 pegloticase pivotal trial participants who did and did not experience acute gout flare during biweekly pegloticase treatment. *noted as comorbidity, not based on BMI. †incudes benign and malignant neoplasms.
Figure. Comorbidities in phase 3 pegloticase pivotal trial participants who did and did not experience acute gout flare during biweekly pegloticase treatment. *noted as comorbidity, not based on BMI. †incudes benign and malignant neoplasms.
Disclosures: N. Schlesinger, Olatec Therapeutics, Novartis, Horizon Pharma, sobi, JW Pharma, LG Chem; L. Padnick-Silver, Horizon Therapeutics; K. Obermeyer, Horizon Therapeutics; B. LaMoreaux, Horizon Therapeutics.
Background/Purpose: Acute gout flare often accompanies urate-lowering therapy initiation and is the most common adverse event associated with pegloticase, a recombinant pegylated uricase that rapidly lowers serum uric acid (sUA).1 A recent case series of uncontrolled gout patients undergoing pegloticase plus methotrexate co-therapy found that patients who experienced acute gout flare during therapy were slightly older, more often obese, and had higher comorbidity burden at baseline than those that did not.2 To better understand these findings, this analysis examined and compared phase 3 pegloticase trial participants with and without gout flare during treatment.
Methods: Patients with uncontrolled gout, sUA≥8 mg/dL, and allopurinol intolerance/recalcitrance who were administered biweekly 8 mg pegloticase infusions as part of the pegloticase phase 3 pivotal trials1 were included. Patient baseline characteristics were compared in this post hoc analysis between participants who did and did not experience ≥1 acute gout flare during treatment.
Results: 66 patients (80.3% male, 57.0±15.12 years old, BMI: 33.0±7.42 kg/m2; sUA: 10.2±1.81 mg/dL) with ≥1 acute gout flare and 18 patients (77.8% males, 53.2±17.40 years old, BMI: 32.8±7.37 kg/m2; sUA=9.7±2.71 mg/dL) without acute gout flare were included. Patients who experienced ≥1 flare following pegloticase initiation had more acute gout flares in the 18 months prior to treatment (11.1±11.49 vs. 5.4±3.31 flares, p=0.040). Though many characteristics were similar, a larger proportion of flare patients were white (69.7% vs. 44.4%, p=0.057). 73.9% (207/280) of listed comorbidities were more prevalent in patients with ≥1 acute gout flare, indicating higher overall comorbidity burden; most notably, diabetes mellitus (30.3% vs. 16.7%; non-insulin dependent: 16.7% vs. 0%), renal failure (25.8% vs. 16.7%), and nervous system disorders (40.9% vs. 16.7%), but all p >0.05 (Figure). Additionally, more patients who experienced acute gout flares during treatment had insomnia (18.2% vs. 0) and depression (15.2% vs. 0) prior to treatment.
Conclusion: Findings of this post hoc analysis largely agree with those of a recent retrospective chart review study2 suggesting higher comorbidity in patients who experience acute gout flares during pegloticase therapy. We also found possible differences in patient race and pre-treatment flare rate. This small study is hypothesis generating and further study is needed on a larger population. However, identifying possible risk factors for acute gout flare following ULT initiation would be beneficial for both patient and physician.
References:
1. Sundy JS, et al. JAMA 2011, 306:711-20.
2. Albert J, et al. Ann Rheum Dis 2022:81(Suppl 1):1644.
 Figure. Comorbidities in phase 3 pegloticase pivotal trial participants who did and did not experience acute gout flare during biweekly pegloticase treatment. *noted as comorbidity, not based on BMI. †incudes benign and malignant neoplasms.
Figure. Comorbidities in phase 3 pegloticase pivotal trial participants who did and did not experience acute gout flare during biweekly pegloticase treatment. *noted as comorbidity, not based on BMI. †incudes benign and malignant neoplasms.Disclosures: N. Schlesinger, Olatec Therapeutics, Novartis, Horizon Pharma, sobi, JW Pharma, LG Chem; L. Padnick-Silver, Horizon Therapeutics; K. Obermeyer, Horizon Therapeutics; B. LaMoreaux, Horizon Therapeutics.

