Back
Poster Session A
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (0150–0180) Muscle Biology, Myositis and Myopathies Poster I
0162: Clinical Features Associated with the Presence of anti-SSA/Ro60 Antibodies in anti-Jo-1 Antibody-positive Myositis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- KY
Koichi Yamaguchi, MD, PhD
Gunma University Graduate School of Medicine
Pittsburgh, PA, United States
Abstract Poster Presenter(s)
Koichi Yamaguchi1, Qi Tang2, Paul Poland3, Rohit Aggarwal4, Chester Oddis5 and Dana Ascherman5, 1Gunma University Graduate School of Medicine, Maebashi, Japan, 2Second Xiangya Hospital of Central South University, Changsha, China, 3University of Pittsburgh School of Medicine, Pittsburgh, PA, 4Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, 5University of Pittsburgh, Pittsburgh, PA
Background/Purpose: Anti-SSA antibodies target two unrelated proteins, Ro52 (E3 ligase) and Ro60 (RNA binding protein), that have different subcellular locations. Previous studies have indicated that antibodies recognizing Ro52 are frequently associated with various myositis-specific autoantibodies (MSAs)--including anti-tRNA synthetase antibodies—and that the coexistence of MSAs and anti-Ro52 antibodies correlates with more aggressive clinical features such as progressive ILD. Although not well-described in the setting of myositis, work from our animal model of HRS (histidyl-tRNA synthetase)-induced myositis suggests that anti-Ro60 antibodies may also be linked to specific MSAs such as anti-Jo-1. We therefore aimed to demonstrate the prevalence and clinical characteristics of Ro60 antibody positivity in patients possessing Jo-1 antibodies.
Methods: This retrospective study investigated 184 patients with anti-Jo-1 antibodies enrolled in the University of Pittsburgh longitudinal myositis registry between 1976 and 2019. We assessed levels of antibodies against recombinant human Ro60 as well as human HRS (Jo-1) using standard solid phase ELISA according to previously established protocols. Background adjusted OD450 levels were converted to standardized units using reference sera of know specificity for either HRS or Ro60. We established a cutoff for Ro60 antibody positivity based on parallel measurements in healthy controls and then compared serum anti-Ro60 antibody levels between patients with anti-Jo-1 antibodies and selected control groups (healthy controls, patients with non-Jo1 synthetase, SRP, or topoisomerase I antibodies). Finally, we assessed the statistical associations between the presence versus absence of Ro60 antibodies and baseline clinical characteristics of anti-Jo1 antibody-positive patients.
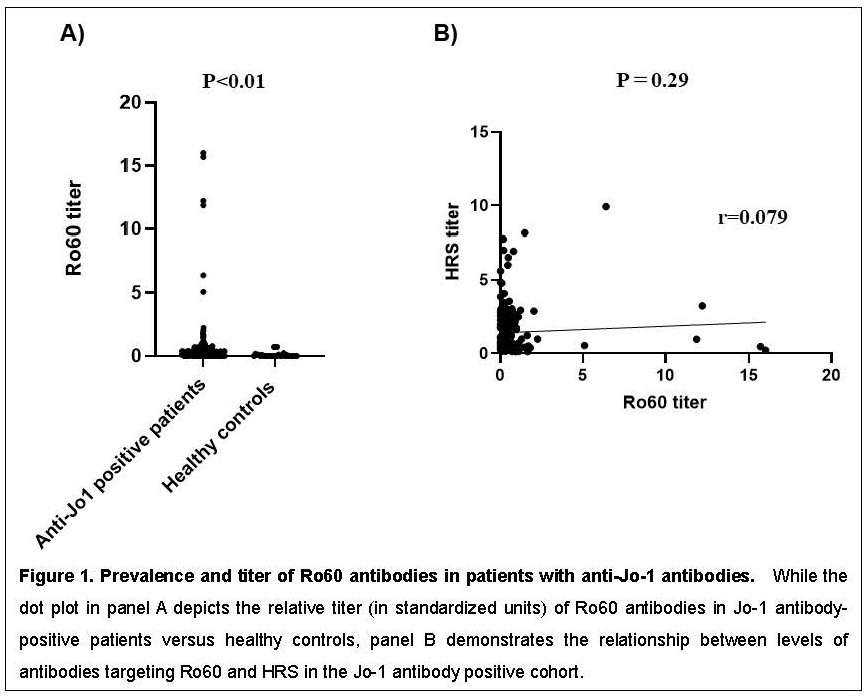
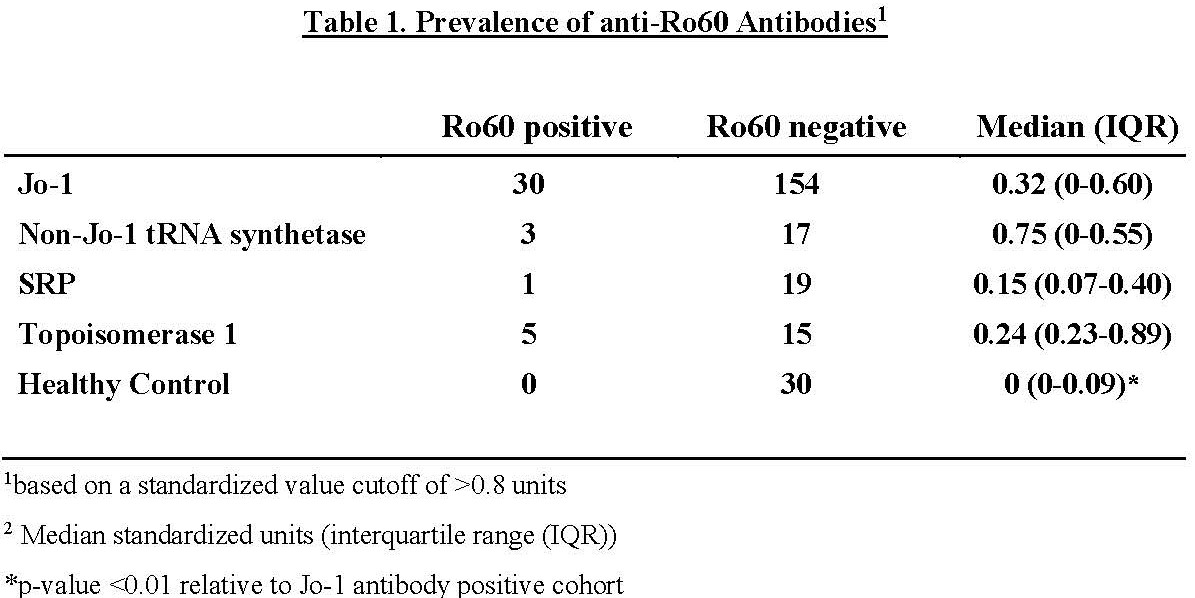
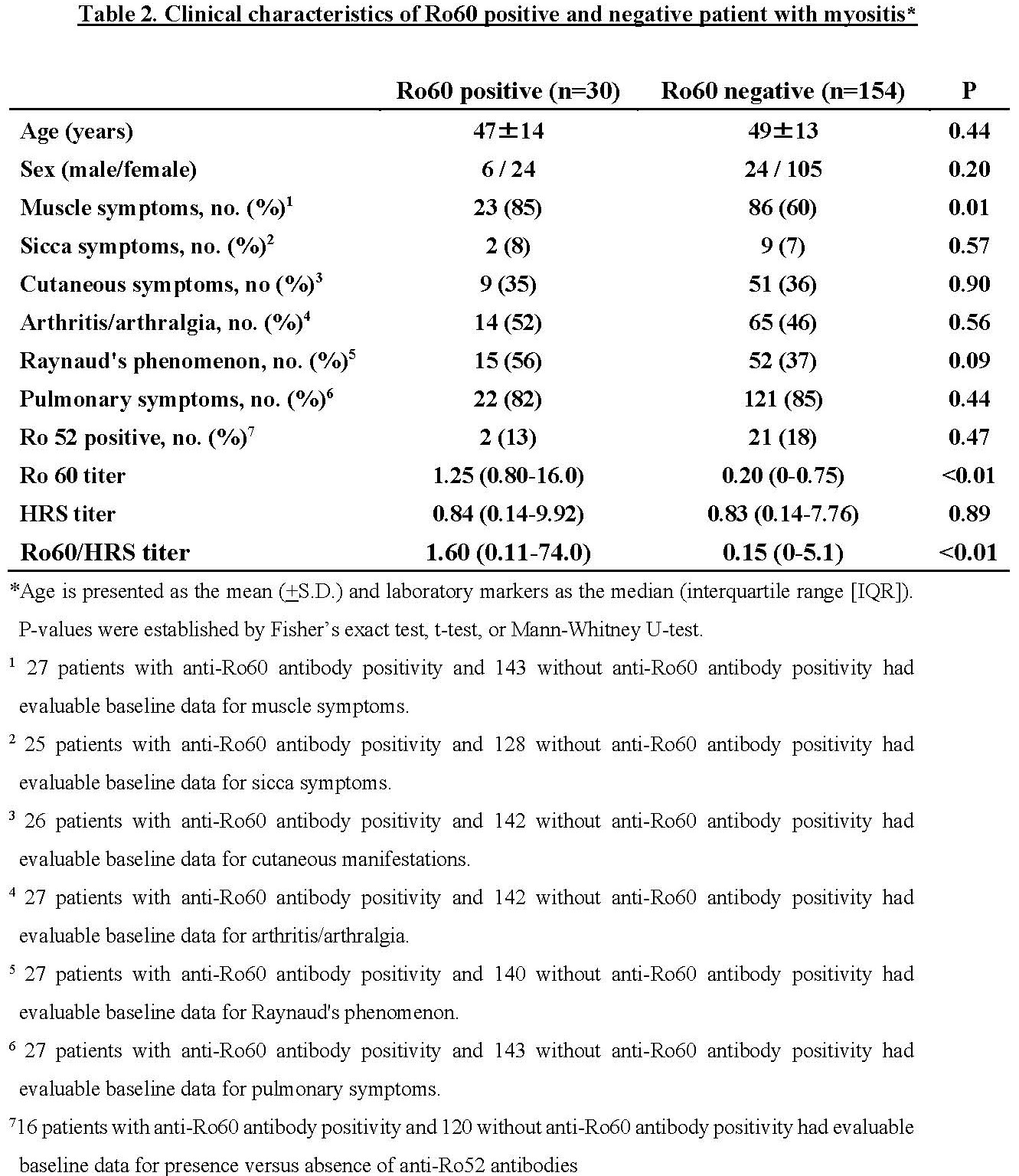
Results: Table 1 demonstrates the relative prevalence of anti-Ro60 antibodies in healthy controls versus patients with anti-Jo-1, non-Jo-1 synthetase, SRP, and topoisomerase I antibodies, using a cutoff of 0.8 standard units. While there was a statistically significant increase in anti-Ro60 levels in Jo-1 antibody positive patients versus healthy controls (p< 0.01, Figure 1A), there was no correlation between Ro60 and Ro52 antibody positivity (dual positive : single positive=0.06) or between levels of Ro60 and HRS antibodies (p=0.29, Figure 1B). Additional statistical analysis indicated that Jo-1 antibody positive patients with Ro60 antibodies had a higher prevalence of myositis (p=0.01) and exhibited a trend towards higher prevalence of Raynaud’s phenomenon (p=0.09) compared to Jo-1 antibody positive patients without Ro60 antibodies (Table 2).
Conclusion: In our large university-based longitudinal myositis cohort, anti-Ro60 antibodies were present in approximately 17% of individuals with anti-Jo-1 antibodies and correlated with greater prevalence of muscle involvement as well as a trend towards greater prevalence of Raynaud’s phenomenon. Based on these considerations, anti-Ro60 antibodies likely represent a new class of prognostically significant myositis-associated antibodies with distinct clinical associations.
Disclosures: K. Yamaguchi, None; Q. Tang, None; P. Poland, None; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; C. Oddis, None; D. Ascherman, None.
Background/Purpose: Anti-SSA antibodies target two unrelated proteins, Ro52 (E3 ligase) and Ro60 (RNA binding protein), that have different subcellular locations. Previous studies have indicated that antibodies recognizing Ro52 are frequently associated with various myositis-specific autoantibodies (MSAs)--including anti-tRNA synthetase antibodies—and that the coexistence of MSAs and anti-Ro52 antibodies correlates with more aggressive clinical features such as progressive ILD. Although not well-described in the setting of myositis, work from our animal model of HRS (histidyl-tRNA synthetase)-induced myositis suggests that anti-Ro60 antibodies may also be linked to specific MSAs such as anti-Jo-1. We therefore aimed to demonstrate the prevalence and clinical characteristics of Ro60 antibody positivity in patients possessing Jo-1 antibodies.
Methods: This retrospective study investigated 184 patients with anti-Jo-1 antibodies enrolled in the University of Pittsburgh longitudinal myositis registry between 1976 and 2019. We assessed levels of antibodies against recombinant human Ro60 as well as human HRS (Jo-1) using standard solid phase ELISA according to previously established protocols. Background adjusted OD450 levels were converted to standardized units using reference sera of know specificity for either HRS or Ro60. We established a cutoff for Ro60 antibody positivity based on parallel measurements in healthy controls and then compared serum anti-Ro60 antibody levels between patients with anti-Jo-1 antibodies and selected control groups (healthy controls, patients with non-Jo1 synthetase, SRP, or topoisomerase I antibodies). Finally, we assessed the statistical associations between the presence versus absence of Ro60 antibodies and baseline clinical characteristics of anti-Jo1 antibody-positive patients.
Results: Table 1 demonstrates the relative prevalence of anti-Ro60 antibodies in healthy controls versus patients with anti-Jo-1, non-Jo-1 synthetase, SRP, and topoisomerase I antibodies, using a cutoff of 0.8 standard units. While there was a statistically significant increase in anti-Ro60 levels in Jo-1 antibody positive patients versus healthy controls (p< 0.01, Figure 1A), there was no correlation between Ro60 and Ro52 antibody positivity (dual positive : single positive=0.06) or between levels of Ro60 and HRS antibodies (p=0.29, Figure 1B). Additional statistical analysis indicated that Jo-1 antibody positive patients with Ro60 antibodies had a higher prevalence of myositis (p=0.01) and exhibited a trend towards higher prevalence of Raynaud’s phenomenon (p=0.09) compared to Jo-1 antibody positive patients without Ro60 antibodies (Table 2).
Conclusion: In our large university-based longitudinal myositis cohort, anti-Ro60 antibodies were present in approximately 17% of individuals with anti-Jo-1 antibodies and correlated with greater prevalence of muscle involvement as well as a trend towards greater prevalence of Raynaud’s phenomenon. Based on these considerations, anti-Ro60 antibodies likely represent a new class of prognostically significant myositis-associated antibodies with distinct clinical associations.



Disclosures: K. Yamaguchi, None; Q. Tang, None; P. Poland, None; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; C. Oddis, None; D. Ascherman, None.

