Back
Poster Session A
Epidemiology, health policy and outcomes
Session: (0187–0213) Patient Outcomes, Preferences, and Attitudes Poster I
0209: Sensitivity of Three Skin-Specific Efficacy Outcomes to Detect Patient- and Physician-Reported Improvement in Overall Skin Disease in Dermatomyositis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JD
Joshua Dan, BA
Icahn School of Medicine at Mount Sinai
New York, NY, United States
Abstract Poster Presenter(s)
Josh Dan1, Grant Sprow2, Josef Concha3, Nilesh Kodali4, DeAnna Diaz5, Thomas Vazquez6, Felix Chin7, Barbara White8 and Victoria Werth3, 1Philadelphia VAMC, Philadelphia, PA, USA and Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, 2Albert Einstein College of Medicine, Philadelphia, PA, 3University of Pennsylvania, Philadelphia, PA, 4New Jersey Medical School, Coppell, TX, 5Philadelphia College of Medicine, Philadelphia, PA, 6FIU Wertheim College of Medicine, Virginia Beach, VA, 7University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, 8SFJ Pharmaceuticals, Towson, MD
Background/Purpose: Variations of the Investigator Global Assessment (IGA) of overall skin disease have been successfully used as a primary efficacy endpoint in registrational clinical trials in skin diseases such as atopic dermatitis and psoriasis, but have not been previously tested in trials in dermatomyositis (DM). Our hypothesis was that change in a DM-specific IGA would reflect patient- and physician-reported improvement in overall skin disease in DM, but not as well as change in Cutaneous Dermatomyositis Disease Activity and Severity Index Activity score (CDASI-A), a validated marker of skin disease activity in DM. The aim of this study was to evaluate the performance of the IGA and CDASI-A in a Phase 3 clinical trial in DM, by evaluating how changes in these efficacy endpoints and the skin-specific symptoms outcome Skindex-29 Symptoms score (Skindex-S) reflected patient- and physician-reported overall improvement in DM skin disease at 1 year in a clinical trial.
Methods: A 5-point DM-specific IGA, CDASI-A, and Skindex-S scores were collected at baseline and Week 52 in a multicenter, randomized, placebo-controlled Phase 3 trial testing efficacy of lenabasum, a cannabinoid receptor type 2 agonist, in DM. At Week 52, both subjects and physicians also reported the degree of improvement in the subject's overall skin disease since study start as "none", "slight", "moderate", or "major", using Patient Improvement Questionnaires (PIQs). Data were analyzed without regard to treatment assignment, because statistical significance was not achieved in the primary efficacy endpoint of change in Total Improvement Score. Mean IGA, CDASI-A, and Skindex-S scores were calculated for subsets of subjects who had different degrees of improvement on PIQs at Week 52. Differences in means in these endpoints for each degree of improvement were compared using Student's t test.
Results: 101 subjects with a mean age of 53 years completed 52 weeks of this study. Mean skin-disease baseline scores were: IGA = 2.8; CDASI-A = 26.4; Skindex-S = 42.2. Changes in mean IGA, CDASI-A, and Skindex-S associated with different degrees of improvement in PIQs, as reported by both subjects and physicians, are presented in Table 1. Change in CDASI-A was the only outcome showed statistically significant greater improvement in subjects with slight versus no improvement and moderate versus slight improvement in overall skin disease, as reported by both subjects and physicians. None of the 3 outcomes showed a statistically significant greater improvement in subjects with moderate versus major improvement. The range of mean change IGA (-0.2 to -1.2) was numerically small across all categories of improvement.
Conclusion: Of the 3 outcomes, change in CDASI-A was best at discriminating improvement at the level of minimal important differences (slight improvement) versus no improvement and discriminating moderate versus slight improvement in overall skin disease. All 3 outcomes showed a ceiling effect, although this was less apparent with CDASI-A and Skindex-S than IGA for physician-reported improvement. The DM-specific IGA used in this study performed the least well of the 3 skin-specific outcomes, which limits its usefulness as an efficacy measure in clinical trials in DM.
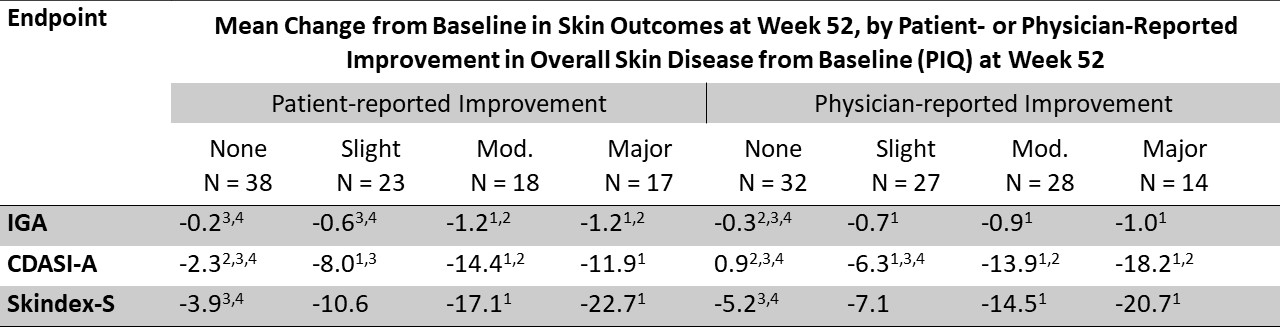 Table 1. Mean Change from Baseline in Skin Efficacy Outcomes at Week 52, by Category of Patient- or Physician-Reported Improvement in Overall Skin Disease from Baseline at Week 52
Table 1. Mean Change from Baseline in Skin Efficacy Outcomes at Week 52, by Category of Patient- or Physician-Reported Improvement in Overall Skin Disease from Baseline at Week 52
1 p ≤ 0.05 for improvement versus none. 2 p ≤ 0.05 for improvement versus slight. 3 p ≤ 0.05 for improvement versus moderate. 4 p ≤ 0.05 for improvement versus major.
Disclosures: J. Dan, None; G. Sprow, None; J. Concha, None; N. Kodali, None; D. Diaz, None; T. Vazquez, None; F. Chin, None; B. White, Corbus Pharmaceuticals; V. Werth, Celgene, Medimmune, Resolve, Genentech, Idera, Janssen, Lilly, Biogen, Bristol Myers Squibb, Gilead, Amgen, Medscape, Nektar, Incyte, EMD Serono, CSL Behring, Principia, Crisalis, Viela Bio, Argenx, Kirin, AstraZeneca, AbbVie, GSK, Cugene, UCB, Beacon Bioscience, Corcept, Gilead, Lupus Research Alliance/BMS.
Background/Purpose: Variations of the Investigator Global Assessment (IGA) of overall skin disease have been successfully used as a primary efficacy endpoint in registrational clinical trials in skin diseases such as atopic dermatitis and psoriasis, but have not been previously tested in trials in dermatomyositis (DM). Our hypothesis was that change in a DM-specific IGA would reflect patient- and physician-reported improvement in overall skin disease in DM, but not as well as change in Cutaneous Dermatomyositis Disease Activity and Severity Index Activity score (CDASI-A), a validated marker of skin disease activity in DM. The aim of this study was to evaluate the performance of the IGA and CDASI-A in a Phase 3 clinical trial in DM, by evaluating how changes in these efficacy endpoints and the skin-specific symptoms outcome Skindex-29 Symptoms score (Skindex-S) reflected patient- and physician-reported overall improvement in DM skin disease at 1 year in a clinical trial.
Methods: A 5-point DM-specific IGA, CDASI-A, and Skindex-S scores were collected at baseline and Week 52 in a multicenter, randomized, placebo-controlled Phase 3 trial testing efficacy of lenabasum, a cannabinoid receptor type 2 agonist, in DM. At Week 52, both subjects and physicians also reported the degree of improvement in the subject's overall skin disease since study start as "none", "slight", "moderate", or "major", using Patient Improvement Questionnaires (PIQs). Data were analyzed without regard to treatment assignment, because statistical significance was not achieved in the primary efficacy endpoint of change in Total Improvement Score. Mean IGA, CDASI-A, and Skindex-S scores were calculated for subsets of subjects who had different degrees of improvement on PIQs at Week 52. Differences in means in these endpoints for each degree of improvement were compared using Student's t test.
Results: 101 subjects with a mean age of 53 years completed 52 weeks of this study. Mean skin-disease baseline scores were: IGA = 2.8; CDASI-A = 26.4; Skindex-S = 42.2. Changes in mean IGA, CDASI-A, and Skindex-S associated with different degrees of improvement in PIQs, as reported by both subjects and physicians, are presented in Table 1. Change in CDASI-A was the only outcome showed statistically significant greater improvement in subjects with slight versus no improvement and moderate versus slight improvement in overall skin disease, as reported by both subjects and physicians. None of the 3 outcomes showed a statistically significant greater improvement in subjects with moderate versus major improvement. The range of mean change IGA (-0.2 to -1.2) was numerically small across all categories of improvement.
Conclusion: Of the 3 outcomes, change in CDASI-A was best at discriminating improvement at the level of minimal important differences (slight improvement) versus no improvement and discriminating moderate versus slight improvement in overall skin disease. All 3 outcomes showed a ceiling effect, although this was less apparent with CDASI-A and Skindex-S than IGA for physician-reported improvement. The DM-specific IGA used in this study performed the least well of the 3 skin-specific outcomes, which limits its usefulness as an efficacy measure in clinical trials in DM.
 Table 1. Mean Change from Baseline in Skin Efficacy Outcomes at Week 52, by Category of Patient- or Physician-Reported Improvement in Overall Skin Disease from Baseline at Week 52
Table 1. Mean Change from Baseline in Skin Efficacy Outcomes at Week 52, by Category of Patient- or Physician-Reported Improvement in Overall Skin Disease from Baseline at Week 521 p ≤ 0.05 for improvement versus none. 2 p ≤ 0.05 for improvement versus slight. 3 p ≤ 0.05 for improvement versus moderate. 4 p ≤ 0.05 for improvement versus major.
Disclosures: J. Dan, None; G. Sprow, None; J. Concha, None; N. Kodali, None; D. Diaz, None; T. Vazquez, None; F. Chin, None; B. White, Corbus Pharmaceuticals; V. Werth, Celgene, Medimmune, Resolve, Genentech, Idera, Janssen, Lilly, Biogen, Bristol Myers Squibb, Gilead, Amgen, Medscape, Nektar, Incyte, EMD Serono, CSL Behring, Principia, Crisalis, Viela Bio, Argenx, Kirin, AstraZeneca, AbbVie, GSK, Cugene, UCB, Beacon Bioscience, Corcept, Gilead, Lupus Research Alliance/BMS.

