Back
Poster Session A
Diversity, inclusion and racial disparities
Session: (0085–0122) Healthcare Disparities in Rheumatology Poster
0113: Escalation to Biologics After Methotrexate Among US Veterans with Rheumatoid Arthritis Living in Rural versus Urban Areas
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AN
Anisha Naik, MBBS, MPH
VA Puget Sound
Seattle, WA, United States
Abstract Poster Presenter(s)
Anisha Naik1, Katherine Wysham2, Bryant England3, Punyasha Roul4, Michael George5, Joshua Baker5, Jennifer Barton6, Jean Liew7, Una Makris8, Gail Kerr9, Grant Cannon10, Ted Mikuls11 and Namrata Singh12, 1VA Puget Sound, Seattle, WA, 2VA Puget Sound/University of Washington, Seattle, WA, 3University of Nebraska Medical Center, Omaha, NE, 4UNMC, Omaha, NE, 5University of Pennsylvania, Philadelphia, PA, 6VA Portland Health Care System/OHSU, Portland, OR, 7Boston University, Boston, MA, 8UT Southwestern Medical Center and Dallas VA, Dallas, TX, 9Washington DC VAMC/Georgetown and Howard Universities, Washington, DC, 10Retired, Salt Lake City, UT, 11Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE, 12University of Washington, Bellevue, WA
Background/Purpose: Timely diagnosis and initiation of appropriate treatments are imperative to improve outcomes for patients with rheumatoid arthritis (RA). Racial and ethnic disparities in RA outcomes are now well recognized [1]. However, whether disparities in healthcare access differ by urban versus rural residence, independent of race, have not been studied. Our objective was to understand how residence in rural versus urban settings impacts biologic disease modifying anti-rheumatic drugs (bDMARD) initiation after methotrexate (MTX) use among Veterans with RA. We hypothesized that rural residence would be associated with lower probability of biologic initiation.
Methods: In this retrospective cohort study utilizing the nationwide U.S. Veterans Affairs databases, we identified adult patients with RA based on presence of a diagnosis code for RA and DMARD use [2]. We included patients receiving an initial prescription of MTX between 2005 and 2014, with data through 2016 used for follow-up and included those with at least 6 months of data prior to receiving a first-ever prescription of MTX (index date). Patients with biologic therapy prescriptions or administration at any time prior to the index date, or those initiating biologic therapy within 30 days after the index date (considered simultaneous MTX and biologic initiation) were excluded. Rural versus urban residence was obtained from VA enrollment data and categorized using the Rural Urban Classification Codes. Our primary outcome of interest was time to biologic initiation within two years of starting MTX. Biologics included were infliximab, adalimumab, etanercept, certolizumab, golimumab, abatacept, rituximab, or tocilizumab. Multivariable Cox proportional hazards models were conducted adjusting for age, sex, race, comorbidities measured using the Charlson comorbidity score, and markers of RA severity (anti-CCP positivity). Censoring occurred at death, end of follow-up (2016), or two years after starting MTX, whichever was earliest.
Results: We included 17,395 veterans with RA (88.3% male, 41.6% with rural residence) and of these, 3,263 (18.8%) initiated a biologic within the first two years of follow up (Table 1). In multivariable models, residence in an urban area was associated with a slightly higher biologic use compared to rural areas (adjusted hazard ratio, aHR 1.08, 95% CI 1.00-1.16) (Table 2). Older age ( >80 years) and non-White race were associated with lower biologic use independent of rural/urban status.
Conclusion: Our study found only modest differences in access to biologic therapies among rural versus urban residing RA patients within the VA healthcare system. Residual confounding may also be present. These findings suggest that disparities are not easily explained by rurality within the VA healthcare system.
References 1. Yip K, Navarro-Millan I: Racial, ethnic, and healthcare disparities in rheumatoid arthritis. Curr Opin Rheumatol 2021, 33(2):117-121.
2. Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, Solomon DH: Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis research & therapy 2011, 13(1):R32.
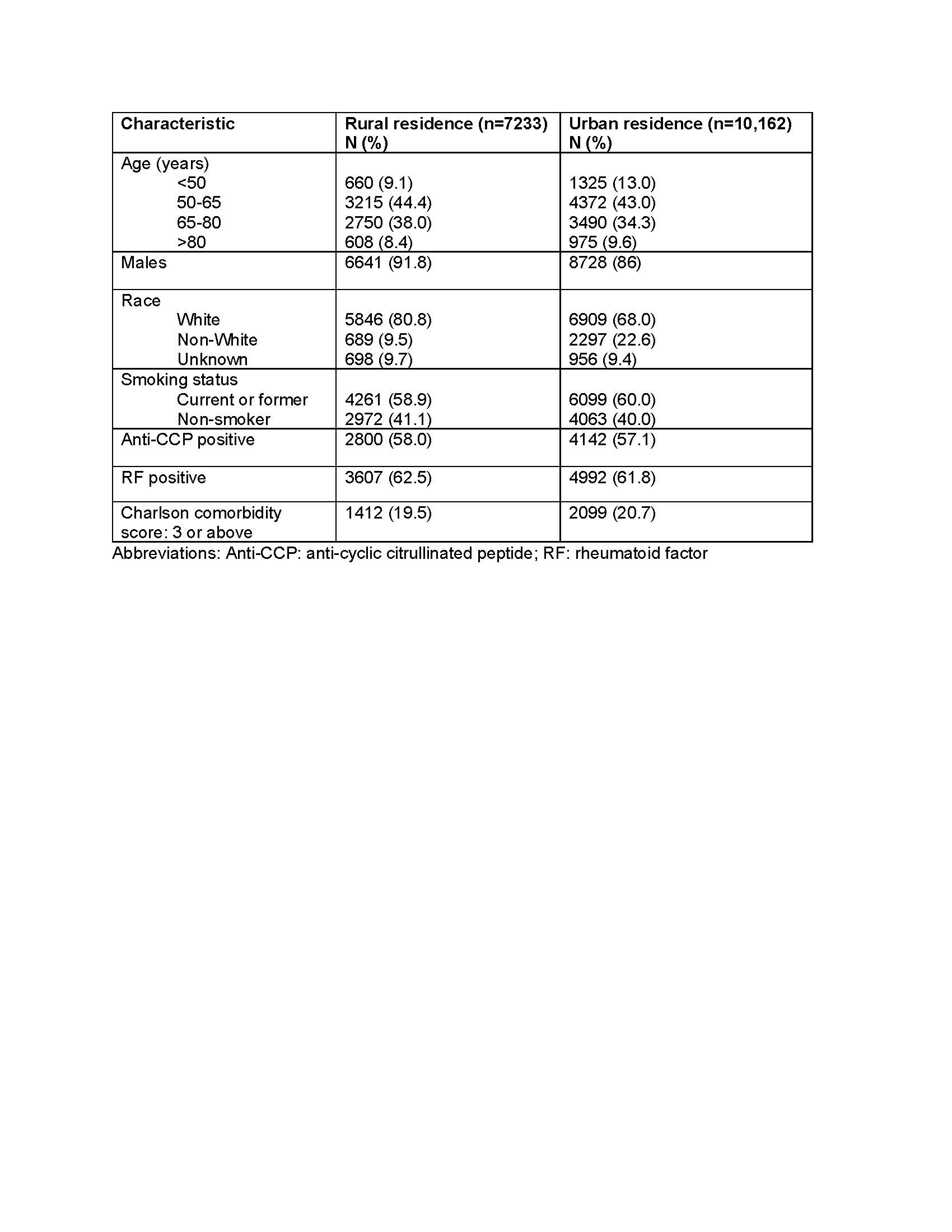 Table 1. Baseline characteristics stratified by residence in a rural versus urban setting.
Table 1. Baseline characteristics stratified by residence in a rural versus urban setting.
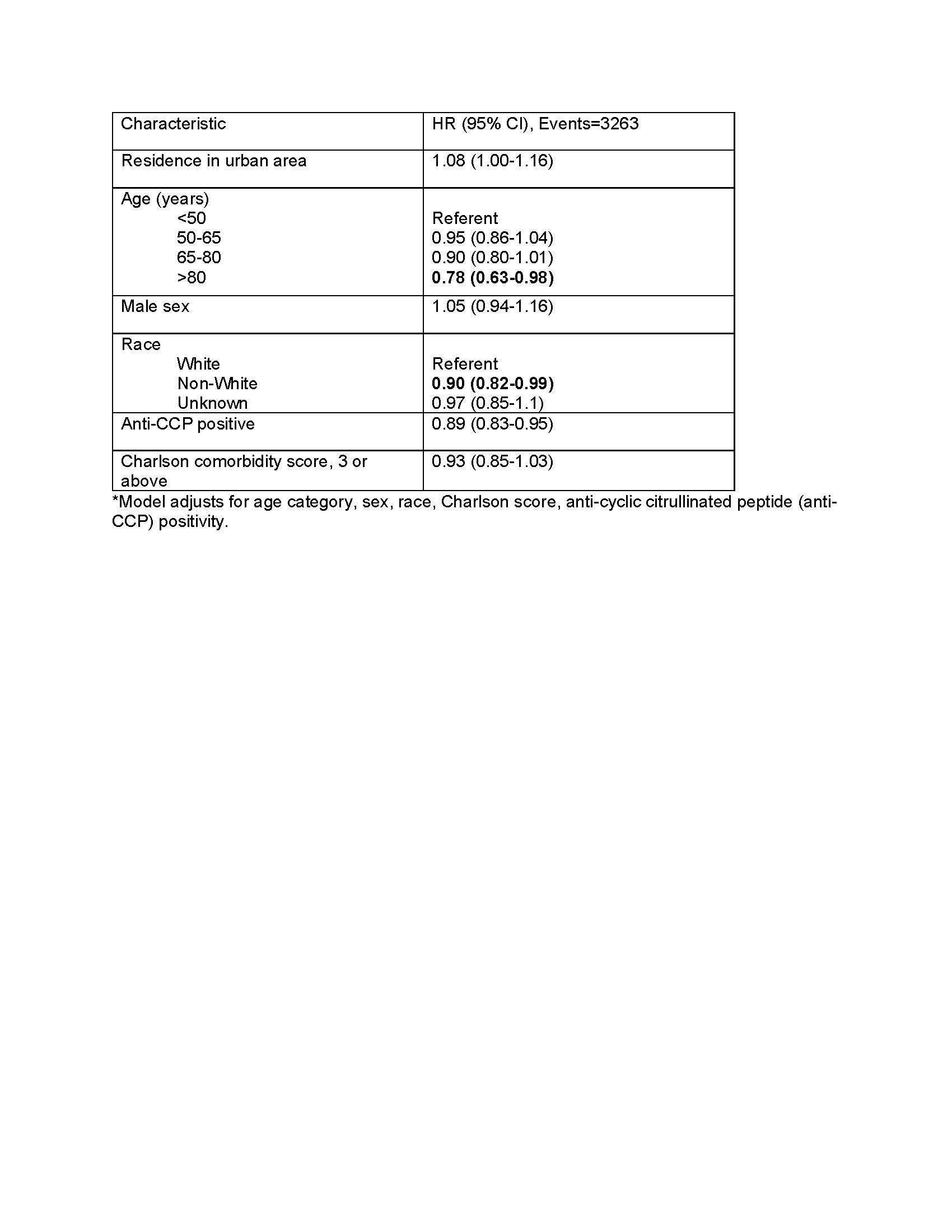 Table 2. Factors associated with time to biologic therapy initiation in patients with RA starting methotrexate, using multivariable Cox regression*.
Table 2. Factors associated with time to biologic therapy initiation in patients with RA starting methotrexate, using multivariable Cox regression*.
Disclosures: A. Naik, None; K. Wysham, None; B. England, Boehringer-Ingelheim; P. Roul, None; M. George, AbbVie, GlaxoSmithKlein(GSK), Chemocentryx; J. Baker, Bristol-Myers Squibb(BMS), RediTrex, Pfizer; J. Barton, None; J. Liew, None; U. Makris, None; G. Kerr, Pfizer, Janssen; G. Cannon, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; N. Singh, None.
Background/Purpose: Timely diagnosis and initiation of appropriate treatments are imperative to improve outcomes for patients with rheumatoid arthritis (RA). Racial and ethnic disparities in RA outcomes are now well recognized [1]. However, whether disparities in healthcare access differ by urban versus rural residence, independent of race, have not been studied. Our objective was to understand how residence in rural versus urban settings impacts biologic disease modifying anti-rheumatic drugs (bDMARD) initiation after methotrexate (MTX) use among Veterans with RA. We hypothesized that rural residence would be associated with lower probability of biologic initiation.
Methods: In this retrospective cohort study utilizing the nationwide U.S. Veterans Affairs databases, we identified adult patients with RA based on presence of a diagnosis code for RA and DMARD use [2]. We included patients receiving an initial prescription of MTX between 2005 and 2014, with data through 2016 used for follow-up and included those with at least 6 months of data prior to receiving a first-ever prescription of MTX (index date). Patients with biologic therapy prescriptions or administration at any time prior to the index date, or those initiating biologic therapy within 30 days after the index date (considered simultaneous MTX and biologic initiation) were excluded. Rural versus urban residence was obtained from VA enrollment data and categorized using the Rural Urban Classification Codes. Our primary outcome of interest was time to biologic initiation within two years of starting MTX. Biologics included were infliximab, adalimumab, etanercept, certolizumab, golimumab, abatacept, rituximab, or tocilizumab. Multivariable Cox proportional hazards models were conducted adjusting for age, sex, race, comorbidities measured using the Charlson comorbidity score, and markers of RA severity (anti-CCP positivity). Censoring occurred at death, end of follow-up (2016), or two years after starting MTX, whichever was earliest.
Results: We included 17,395 veterans with RA (88.3% male, 41.6% with rural residence) and of these, 3,263 (18.8%) initiated a biologic within the first two years of follow up (Table 1). In multivariable models, residence in an urban area was associated with a slightly higher biologic use compared to rural areas (adjusted hazard ratio, aHR 1.08, 95% CI 1.00-1.16) (Table 2). Older age ( >80 years) and non-White race were associated with lower biologic use independent of rural/urban status.
Conclusion: Our study found only modest differences in access to biologic therapies among rural versus urban residing RA patients within the VA healthcare system. Residual confounding may also be present. These findings suggest that disparities are not easily explained by rurality within the VA healthcare system.
References 1. Yip K, Navarro-Millan I: Racial, ethnic, and healthcare disparities in rheumatoid arthritis. Curr Opin Rheumatol 2021, 33(2):117-121.
2. Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, Solomon DH: Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis research & therapy 2011, 13(1):R32.
 Table 1. Baseline characteristics stratified by residence in a rural versus urban setting.
Table 1. Baseline characteristics stratified by residence in a rural versus urban setting. Table 2. Factors associated with time to biologic therapy initiation in patients with RA starting methotrexate, using multivariable Cox regression*.
Table 2. Factors associated with time to biologic therapy initiation in patients with RA starting methotrexate, using multivariable Cox regression*.Disclosures: A. Naik, None; K. Wysham, None; B. England, Boehringer-Ingelheim; P. Roul, None; M. George, AbbVie, GlaxoSmithKlein(GSK), Chemocentryx; J. Baker, Bristol-Myers Squibb(BMS), RediTrex, Pfizer; J. Barton, None; J. Liew, None; U. Makris, None; G. Kerr, Pfizer, Janssen; G. Cannon, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; N. Singh, None.

