Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0343: Severe Non-adherence to Hydroxychloroquine Is Associated with Flares, Early Damage, and Mortality in Systemic Lupus Erythematosus: Data from 660 Patients from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- YN
Yann Nguyen, MD, PhD
AP-HP.Centre Université Paris Cité Hôpital Cochin
Montmorency, France
Abstract Poster Presenter(s)
Yann Nguyen1, Benoît BLanchet2, Murray Urowitz3, John Hanly4, Caroline Gordon5, Sang-Cheol Bae6, Juanita Romero-Diaz7, Jorge Sanchez-Guerrero8, Ann E Clarke9, Sasha Bernatsky10, Daniel Wallace11, David Isenberg12, Anisur Rahman13, Joan Merrill14, Paul R Fortin15, Dafna Gladman16, Ian N. Bruce17, Michelle Petri18, Ellen M. Ginzler19, Mary Anne Dooley20, Rosalind Ramsey-Goldman21, Susan Manzi22, Andreas Jönsen23, Graciela Alarcón24, Ronald van Vollenhoven25, Cynthia Aranow26, Veronique Le Guern27, Meggan Mackay26, Guillermo Ruiz-Irastorza28, S. Sam Lim29, Murat Inanc30, Kenneth C Kalunian31, Soren Jacobsen32, Christine Peschken33, Diane Kamen34, Anca Askanase35, Jill Buyon36 and Nathalie Costedoat-Chalumeau37, 1AP-HP.Centre Universit Paris Cit Hôpital Cochin, Montmorency, France, 2Biologie du médicament-Toxicologie, AP-HP Centre – Hôpital Cochin, Université Paris Cité, Paris, France, Paris, 3University of Toronto, University Health Network, Schroeder Arthritis Institute, Toronto, ON, Canada, 4Division of Rheumatology, Queen Elizabeth II Health Sciences Center (Nova Scotia Rehabilitation Site) and Dalhousie University, Halifax, NS, Canada, 5Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom, 6Hanyang University Medical Center, Seoul, Republic of Korea, 7Instituto Nacional de Ciencias Medicas y Nutricion SZ, Ciudad de México, Mexico, 8Mount Sinai Hospital and University Health Network, University of Toronto, Toronto, ON, Canada, 9University of Calgary, Division of Rheumatology, Cumming School of Medicine, Calgary, AB, Canada, 10Research Institute of the McGill University Health Centre, Montréal, QC, Canada, 11Cedars-Sinai Medical Center, Los Angeles, CA, 12University College London, London, United Kingdom, 13Centre for Rheumatology, Department of Medicine, University College London, London, United Kingdom, 14Oklahoma Medical Research Foundation, Oklahoma City, OK, 15Centre ARThrite - CHU de Québec - Université Laval, Québec, QC, Canada, 16Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 17Centre for Epidemiology Versus Arthritis, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom, 18Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 19SUNY Downstate Health Sciences University, Department of Medicine, Brooklyn, NY, 20Thurston Arthritis Research Center, University of North Carolina, Chapel Hill, NC, 21Northwestern University Feinberg School of Medicine, Chicago, USA, Chicago, IL, 22Allegheny Health Network, Lupus Center of Excellence, Wexford, PA, 23Lund University, Lund, Sweden, 24The University of Alabama at Birmingham, Oakland, 25Amsterdam University Medical Centers, Amsterdam, Netherlands, 26Feinstein Institutes for Medical Research, Manhasset, NY, 27Hôpital Cochin, Paris, France, 28Autoimmune Diseases Research Unit, Biocruces Bizkaia Health Research Institute, Hospital Universitario Cruces, UPV/EHU, Barakaldo, Spain, 29Emory University, Atlanta, GA, 30Division of Rheumatology, Department of Internal Medicine, Istanbul Medical Faculty, Istanbul University, Istambul, Turkey, 31University of California San Diego School of Medicine, La Jolla, 32Rigshospitalet, Copenhagen, Denmark, 33University of Manitoba, Winnipeg, MB, Canada, 34Medical University of South Carolina, Charleston, SC, 35Columbia University Medical Center, New York, NY, 36NYU Grossman School of Medicine, New York, NY, 37Inserm DR Paris 5, Paris, France
Background/Purpose: The efficacy of antimalarials, especially hydroxychloroquine (HCQ), in preventing flares of systemic lupus erythematosus (SLE) is well demonstrated, but its effectiveness is impaired by non-adherence to treatment, reported to vary between 3% and 85%. We aimed to assess the associations of baseline severe non-adherence to HCQ, objectively assessed by HCQ serum levels, and risks of SLE flares, damage, and mortality over 5 years of follow-up.
Methods: The SLICC Inception Cohort is a multicentric prospective study of SLE patients from 31 centers in 11 countries. Patients were enrolled within 15 months of recognition of SLE (1997 ACR classification criteria). Serum HCQ levels of patients with HCQ prescription for at least 3 months, sampled at enrollment (or, if unavailable, during the first-year follow-up visits), were centrally measured. Severe non-adherence was defined by a serum HCQ level < 106 ng/mL for a daily prescribed HCQ dose of 400 mg, and < 53 ng/mL for a daily HCQ dose of 200 mg, respectively (1). SLE flare was defined by the occurrence of one of the following events within the first year following serum collection: (a) increase of at least four point in the SLEDAI-2K; (b) new start in prednisone (oral or pulse) or immunosuppressive agent; (c) a new renal involvement (active nephritis, nephrotic syndrome). Association between severe non-adherence and SLE flare was assessed with logistic regression models, further adjusted on potential confounders. Damage was assessed by the time until an increase ≥1 in the SLICC damage index (SDI), within the 5 years following HCQ measurement. Associations between severe non-adherence and either damage or mortality (from all cause) within 5 years following HCQ measurement were assessed with Cox proportional hazard models, adjusted on sex, education, and potential confounders.
Results: Of 1849 cohort subjects, 660 patients (88% women) were included. Median [interquartile range] serum HCQ level was 388 ng/mL (244-566) and 48 patients (7.3%) had severe HCQ non-adherence. No factors were clearly associated with severe non-adherence. A SLE flare occurred in 191 (28.9%) patients within the first year (28 [58.3%] non-adherent patients versus 163 [26.6%] other patients). In multivariate analysis, severe nonadherence was independently associated with the risk of flare (OR= 3.32; 95% CI 1.78-6.28).
Within five years, 167 patients (25.3%) had ≥1 point increase of SDI. Severe on-adherence was associated with an increase in the SDI within each of the first 3 years (HR 1.92 at 3 years; 95% CI 1.05–3.50).
Eleven patients died within 5 years, including 3 with severe non-adherence (unadjusted HR 5.41; 95% CI 1.43–20.39).
Conclusion: In this large multicentric international prospective cohort, severe non-adherence was independently associated with the risk of SLE flare in the following year, with early damage, and 5-year mortality. As severe non-adherence is often unknown by the physician and since no predictive clinical or biological factors were identified, our results suggest the benefits of testing to detect severe non-adherence, to identify the patients at risk.
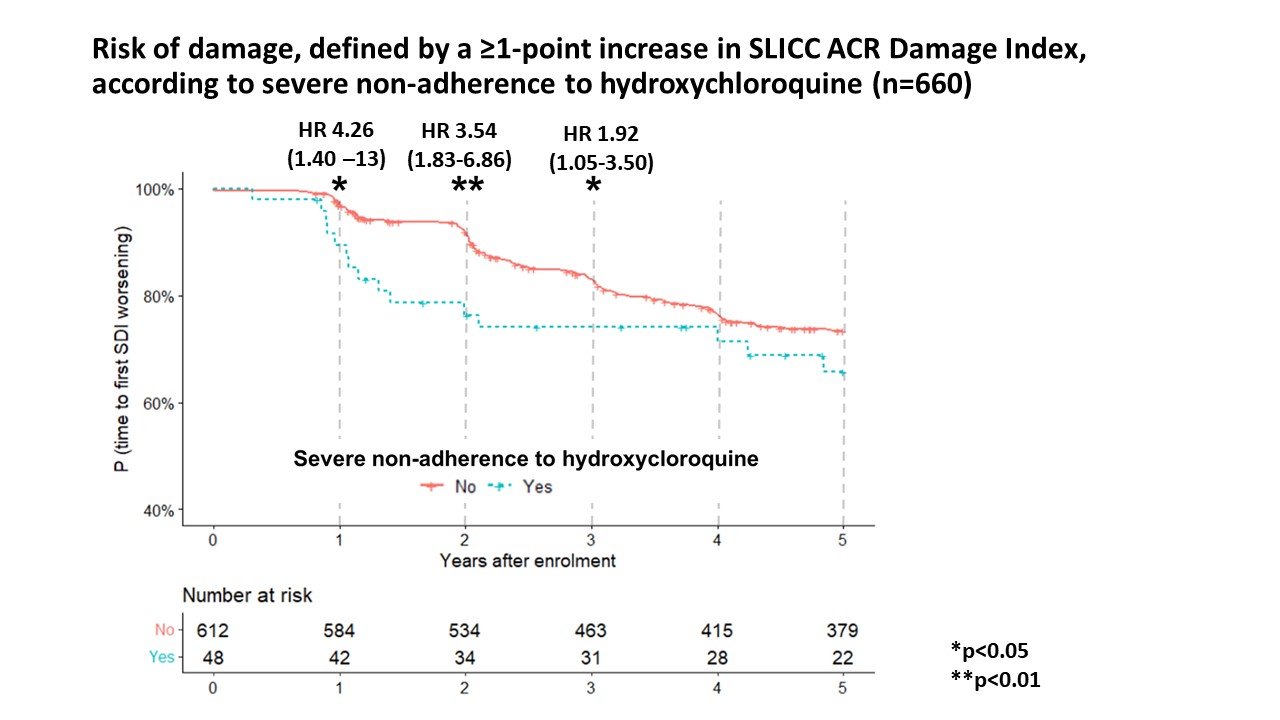 Figure 1. Kaplan Meier curves for the risk of damage, defined by a ≥1-point increase of SLICC/ACR damage index, according to severe non-adherence. Severe non-adherence was associated with the risk of SDI worsening at 1 (adjusted HR 4.26; 95% CI 1.40–13), 2 (adjusted HR 3.54; 95% CI 1.83–6.86) and 3 years after the HCQ measurement (adjusted HR 1.92; 95% CI 1.05–3.50), with a non-significant trend at 5 years (adjusted HR 1.47; 95% CI 0.86–2.49). *P < 0.05; **P < 0.01; NS: non-significant. Following variables were included in the multivariate models: age, black race, education level (post-secondary; ≤High school), SLEDAI-2000, azathioprine, and severe HCQ non-adherence.
Figure 1. Kaplan Meier curves for the risk of damage, defined by a ≥1-point increase of SLICC/ACR damage index, according to severe non-adherence. Severe non-adherence was associated with the risk of SDI worsening at 1 (adjusted HR 4.26; 95% CI 1.40–13), 2 (adjusted HR 3.54; 95% CI 1.83–6.86) and 3 years after the HCQ measurement (adjusted HR 1.92; 95% CI 1.05–3.50), with a non-significant trend at 5 years (adjusted HR 1.47; 95% CI 0.86–2.49). *P < 0.05; **P < 0.01; NS: non-significant. Following variables were included in the multivariate models: age, black race, education level (post-secondary; ≤High school), SLEDAI-2000, azathioprine, and severe HCQ non-adherence.
Disclosures: Y. Nguyen, None; B. BLanchet, None; M. Urowitz, None; J. Hanly, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP; S. Bae, None; J. Romero-Diaz, Biogen; J. Sanchez-Guerrero, None; A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); S. Bernatsky, None; D. Wallace, None; D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; A. Rahman, None; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; P. Fortin, AstraZeneca, GlaxoSmithKlein(GSK); D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; E. Ginzler, Aurinia Pharma; M. Dooley, None; R. Ramsey-Goldman, None; S. Manzi, AstraZeneca, GlaxoSmithKline (GSK), Exagen Diagnostics Inc, AbbVie, HGS, Cugene, Lilly, UCB Advisory Board, Lupus Foundation of America; A. Jönsen, None; G. Alarcón, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; C. Aranow, None; V. Le Guern, None; M. Mackay, None; G. Ruiz-Irastorza, None; S. Lim, None; M. Inanc, None; K. Kalunian, None; S. Jacobsen, None; C. Peschken, None; D. Kamen, None; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); J. Buyon, None; N. Costedoat-Chalumeau, UCB, Roche.
Background/Purpose: The efficacy of antimalarials, especially hydroxychloroquine (HCQ), in preventing flares of systemic lupus erythematosus (SLE) is well demonstrated, but its effectiveness is impaired by non-adherence to treatment, reported to vary between 3% and 85%. We aimed to assess the associations of baseline severe non-adherence to HCQ, objectively assessed by HCQ serum levels, and risks of SLE flares, damage, and mortality over 5 years of follow-up.
Methods: The SLICC Inception Cohort is a multicentric prospective study of SLE patients from 31 centers in 11 countries. Patients were enrolled within 15 months of recognition of SLE (1997 ACR classification criteria). Serum HCQ levels of patients with HCQ prescription for at least 3 months, sampled at enrollment (or, if unavailable, during the first-year follow-up visits), were centrally measured. Severe non-adherence was defined by a serum HCQ level < 106 ng/mL for a daily prescribed HCQ dose of 400 mg, and < 53 ng/mL for a daily HCQ dose of 200 mg, respectively (1). SLE flare was defined by the occurrence of one of the following events within the first year following serum collection: (a) increase of at least four point in the SLEDAI-2K; (b) new start in prednisone (oral or pulse) or immunosuppressive agent; (c) a new renal involvement (active nephritis, nephrotic syndrome). Association between severe non-adherence and SLE flare was assessed with logistic regression models, further adjusted on potential confounders. Damage was assessed by the time until an increase ≥1 in the SLICC damage index (SDI), within the 5 years following HCQ measurement. Associations between severe non-adherence and either damage or mortality (from all cause) within 5 years following HCQ measurement were assessed with Cox proportional hazard models, adjusted on sex, education, and potential confounders.
Results: Of 1849 cohort subjects, 660 patients (88% women) were included. Median [interquartile range] serum HCQ level was 388 ng/mL (244-566) and 48 patients (7.3%) had severe HCQ non-adherence. No factors were clearly associated with severe non-adherence. A SLE flare occurred in 191 (28.9%) patients within the first year (28 [58.3%] non-adherent patients versus 163 [26.6%] other patients). In multivariate analysis, severe nonadherence was independently associated with the risk of flare (OR= 3.32; 95% CI 1.78-6.28).
Within five years, 167 patients (25.3%) had ≥1 point increase of SDI. Severe on-adherence was associated with an increase in the SDI within each of the first 3 years (HR 1.92 at 3 years; 95% CI 1.05–3.50).
Eleven patients died within 5 years, including 3 with severe non-adherence (unadjusted HR 5.41; 95% CI 1.43–20.39).
Conclusion: In this large multicentric international prospective cohort, severe non-adherence was independently associated with the risk of SLE flare in the following year, with early damage, and 5-year mortality. As severe non-adherence is often unknown by the physician and since no predictive clinical or biological factors were identified, our results suggest the benefits of testing to detect severe non-adherence, to identify the patients at risk.
 Figure 1. Kaplan Meier curves for the risk of damage, defined by a ≥1-point increase of SLICC/ACR damage index, according to severe non-adherence. Severe non-adherence was associated with the risk of SDI worsening at 1 (adjusted HR 4.26; 95% CI 1.40–13), 2 (adjusted HR 3.54; 95% CI 1.83–6.86) and 3 years after the HCQ measurement (adjusted HR 1.92; 95% CI 1.05–3.50), with a non-significant trend at 5 years (adjusted HR 1.47; 95% CI 0.86–2.49). *P < 0.05; **P < 0.01; NS: non-significant. Following variables were included in the multivariate models: age, black race, education level (post-secondary; ≤High school), SLEDAI-2000, azathioprine, and severe HCQ non-adherence.
Figure 1. Kaplan Meier curves for the risk of damage, defined by a ≥1-point increase of SLICC/ACR damage index, according to severe non-adherence. Severe non-adherence was associated with the risk of SDI worsening at 1 (adjusted HR 4.26; 95% CI 1.40–13), 2 (adjusted HR 3.54; 95% CI 1.83–6.86) and 3 years after the HCQ measurement (adjusted HR 1.92; 95% CI 1.05–3.50), with a non-significant trend at 5 years (adjusted HR 1.47; 95% CI 0.86–2.49). *P < 0.05; **P < 0.01; NS: non-significant. Following variables were included in the multivariate models: age, black race, education level (post-secondary; ≤High school), SLEDAI-2000, azathioprine, and severe HCQ non-adherence.Disclosures: Y. Nguyen, None; B. BLanchet, None; M. Urowitz, None; J. Hanly, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP; S. Bae, None; J. Romero-Diaz, Biogen; J. Sanchez-Guerrero, None; A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); S. Bernatsky, None; D. Wallace, None; D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; A. Rahman, None; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; P. Fortin, AstraZeneca, GlaxoSmithKlein(GSK); D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; E. Ginzler, Aurinia Pharma; M. Dooley, None; R. Ramsey-Goldman, None; S. Manzi, AstraZeneca, GlaxoSmithKline (GSK), Exagen Diagnostics Inc, AbbVie, HGS, Cugene, Lilly, UCB Advisory Board, Lupus Foundation of America; A. Jönsen, None; G. Alarcón, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; C. Aranow, None; V. Le Guern, None; M. Mackay, None; G. Ruiz-Irastorza, None; S. Lim, None; M. Inanc, None; K. Kalunian, None; S. Jacobsen, None; C. Peschken, None; D. Kamen, None; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); J. Buyon, None; N. Costedoat-Chalumeau, UCB, Roche.

