Back
Poster Session A
Osteoarthritis (OA) and related disorders
Session: (0017–0033) Osteoarthritis and Joint Biology – Basic Science Poster
0019: Bacterial Peptidoglycan in Synovial Tissue at Time of Total Knee Arthroplasty Is Associated with Inflammatory Synovitis and Younger Patient Age
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MH
Meaghan Holub, BS
Central Michigan University College of Medicine
Mount Pleasant, MI, United States
Abstract Poster Presenter(s)
Meaghan Holub1, Amanda Wahhab2, Joseph Rouse2, Brandon Jutras3, Klemen Strle4, Adam Edelstein5 and Robert Lochhead6, 1Central Michigan University College of Medicine, Mount Pleasant, MI, 2Medical College of Wisconsin, Milwaukee, WI, 3Virginia Tech, Blacksburg, VA, 4Wadsworth Center, Albany, NY, 5Northwestern Medicine, Chicago, IL, 6Medical College of Wisconsin, Germantown, WI
Background/Purpose: Peptidoglycan (PG) is a bacterial cell wall component that is known to induce innate immune responses. PG has been identified as a driver of synovial inflammation in patients with Lyme arthritis. Recent studies have also found PG in synovial fluid from patients with inflammatory and degenerative arthritis with no history of clinical infection. It is unknown if PG may be a driver of inflammation in patients undergoing total knee arthroplasty (TKA). The purpose of this study was to determine if PG is present in synovial tissue at time of TKA and if its presence is associated with inflammation and patient reported outcomes.
Methods: Intraoperative synovial tissue and synovial fluid samples were obtained from 56 consecutive patients undergoing primary TKA, none of whom had history of infection. Tissues from 4 unrelated periprosthetic joint infection cases were used as positive controls. PG in synovial tissue was detected by staining of serial sections of 10 mm2 of tissue with anti-PG antiserum using immunohistochemistry (IHC). PG severity was ordinally classified 0 through 4 according to the number of PG foci identified per 10 mm2. Synovial tissue inflammation and fibrosis were assessed by histopathology and synovial fluid cytokine levels were assessed by multiplex assay. Patient demographics and comorbidities were recorded. Knee injury and Osteoarthritis Outcome Score were collected prior to TKA and 3, 6 and 12 months postoperatively. Associations between PG severity scores and synovial inflammation, cytokine levels, and patient reported outcomes were assessed using regression analyses.
Results: Mean patient age was 65.6 ± 12.8 years, mean BMI was 31.9 ± 5.9 kg/m2, and 57% were female. 33/56 (59%) of primary TKA synovial tissue samples were positive for PG by IHC, with a median of 8 PG occurrences per 10 mm2 of tissue in PG-positive samples. Tissue inflammation as assessed by histopathology significantly correlated with PG (r=0.43, p=0.001) and there was a significant correlation between IL-6 levels in synovial fluid and PG (r=0.32, p=0.026). Interestingly, we observed a significant inverse correlation between PG and age at time of TKA (r=-0.29, p=0.033), indicating younger age at time of TKA was associated with higher PG levels. KOOS JR scores improved from 39.3 ± 14.7 at baseline to 77.8 ± 15.5 postoperatively (p=1.91E-19). No associations were found between PG and patient-reported outcomes post TKA.
Conclusion: Peptidoglycan is commonly found in synovial tissue from patients undergoing TKA. Our data indicate that PG may play an important role in inflammatory synovitis, particularly in patients who undergo TKA at a relatively younger age. Further research is needed on PG-induced inflammation and its potential as a therapeutic target.
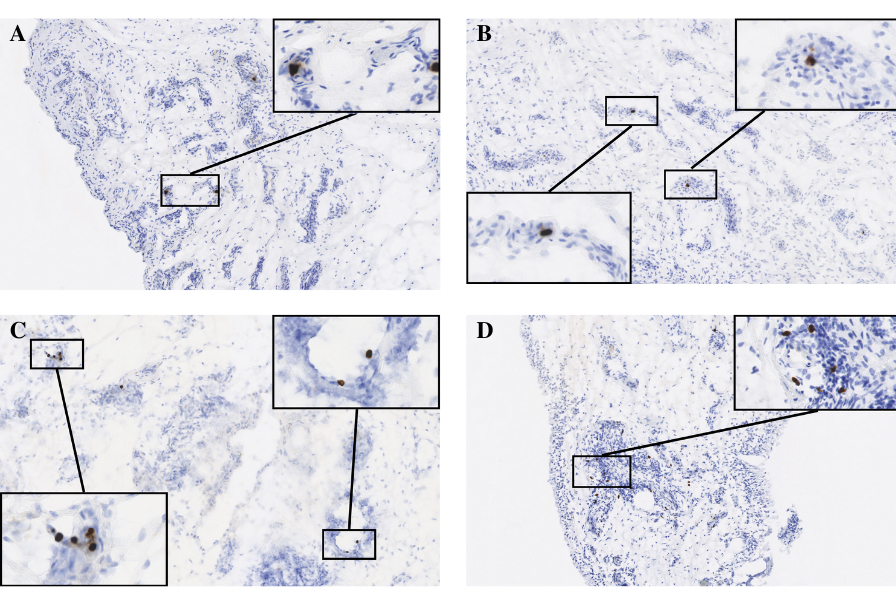 Figure 1: Synovial tissue with abundance of cells positive for bacterial PG (brown) per 10mm2 area was ranked according to a 4-point scale (0=none, 1=1-9 PG foci, 2=10-19 foci, 3=20-29 foci, 4=30+ foci). (A) Positive control – synovial tissue sample obtained from periprosthetic joint infection patient (B) Score 2 – PG positive cells surrounded by foci of inflammatory infiltrate (blue) (C) Score 3 – PG positive cells (brown) show selective accumulation surrounding various points of vasculature in synovial tissue sample (D) Score 4 – abundant accumulation of PG positive cells (brown) in tissue segment containing high-density inflammatory infiltrate (blue) and blood vessels.
Figure 1: Synovial tissue with abundance of cells positive for bacterial PG (brown) per 10mm2 area was ranked according to a 4-point scale (0=none, 1=1-9 PG foci, 2=10-19 foci, 3=20-29 foci, 4=30+ foci). (A) Positive control – synovial tissue sample obtained from periprosthetic joint infection patient (B) Score 2 – PG positive cells surrounded by foci of inflammatory infiltrate (blue) (C) Score 3 – PG positive cells (brown) show selective accumulation surrounding various points of vasculature in synovial tissue sample (D) Score 4 – abundant accumulation of PG positive cells (brown) in tissue segment containing high-density inflammatory infiltrate (blue) and blood vessels.
Disclosures: M. Holub, None; A. Wahhab, None; J. Rouse, None; B. Jutras, None; K. Strle, None; A. Edelstein, None; R. Lochhead, None.
Background/Purpose: Peptidoglycan (PG) is a bacterial cell wall component that is known to induce innate immune responses. PG has been identified as a driver of synovial inflammation in patients with Lyme arthritis. Recent studies have also found PG in synovial fluid from patients with inflammatory and degenerative arthritis with no history of clinical infection. It is unknown if PG may be a driver of inflammation in patients undergoing total knee arthroplasty (TKA). The purpose of this study was to determine if PG is present in synovial tissue at time of TKA and if its presence is associated with inflammation and patient reported outcomes.
Methods: Intraoperative synovial tissue and synovial fluid samples were obtained from 56 consecutive patients undergoing primary TKA, none of whom had history of infection. Tissues from 4 unrelated periprosthetic joint infection cases were used as positive controls. PG in synovial tissue was detected by staining of serial sections of 10 mm2 of tissue with anti-PG antiserum using immunohistochemistry (IHC). PG severity was ordinally classified 0 through 4 according to the number of PG foci identified per 10 mm2. Synovial tissue inflammation and fibrosis were assessed by histopathology and synovial fluid cytokine levels were assessed by multiplex assay. Patient demographics and comorbidities were recorded. Knee injury and Osteoarthritis Outcome Score were collected prior to TKA and 3, 6 and 12 months postoperatively. Associations between PG severity scores and synovial inflammation, cytokine levels, and patient reported outcomes were assessed using regression analyses.
Results: Mean patient age was 65.6 ± 12.8 years, mean BMI was 31.9 ± 5.9 kg/m2, and 57% were female. 33/56 (59%) of primary TKA synovial tissue samples were positive for PG by IHC, with a median of 8 PG occurrences per 10 mm2 of tissue in PG-positive samples. Tissue inflammation as assessed by histopathology significantly correlated with PG (r=0.43, p=0.001) and there was a significant correlation between IL-6 levels in synovial fluid and PG (r=0.32, p=0.026). Interestingly, we observed a significant inverse correlation between PG and age at time of TKA (r=-0.29, p=0.033), indicating younger age at time of TKA was associated with higher PG levels. KOOS JR scores improved from 39.3 ± 14.7 at baseline to 77.8 ± 15.5 postoperatively (p=1.91E-19). No associations were found between PG and patient-reported outcomes post TKA.
Conclusion: Peptidoglycan is commonly found in synovial tissue from patients undergoing TKA. Our data indicate that PG may play an important role in inflammatory synovitis, particularly in patients who undergo TKA at a relatively younger age. Further research is needed on PG-induced inflammation and its potential as a therapeutic target.
 Figure 1: Synovial tissue with abundance of cells positive for bacterial PG (brown) per 10mm2 area was ranked according to a 4-point scale (0=none, 1=1-9 PG foci, 2=10-19 foci, 3=20-29 foci, 4=30+ foci). (A) Positive control – synovial tissue sample obtained from periprosthetic joint infection patient (B) Score 2 – PG positive cells surrounded by foci of inflammatory infiltrate (blue) (C) Score 3 – PG positive cells (brown) show selective accumulation surrounding various points of vasculature in synovial tissue sample (D) Score 4 – abundant accumulation of PG positive cells (brown) in tissue segment containing high-density inflammatory infiltrate (blue) and blood vessels.
Figure 1: Synovial tissue with abundance of cells positive for bacterial PG (brown) per 10mm2 area was ranked according to a 4-point scale (0=none, 1=1-9 PG foci, 2=10-19 foci, 3=20-29 foci, 4=30+ foci). (A) Positive control – synovial tissue sample obtained from periprosthetic joint infection patient (B) Score 2 – PG positive cells surrounded by foci of inflammatory infiltrate (blue) (C) Score 3 – PG positive cells (brown) show selective accumulation surrounding various points of vasculature in synovial tissue sample (D) Score 4 – abundant accumulation of PG positive cells (brown) in tissue segment containing high-density inflammatory infiltrate (blue) and blood vessels. Disclosures: M. Holub, None; A. Wahhab, None; J. Rouse, None; B. Jutras, None; K. Strle, None; A. Edelstein, None; R. Lochhead, None.

