Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0317–0342) SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
0321: M-Phase Phosphoprotein 1 (MPP-1) Autoantibodies as a Potential Biomarker for Cranial Neuropathies in an International SLE Inception Cohort
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- EK
Eugene Krustev, MD
University of Calgary
Calgary, AB, Canada
Abstract Poster Presenter(s)
Eugene Krustev1, John Hanly2, Ricky Chin3, Katherine Buhler1, Francesca S Cardwell4, Murray Urowitz5, Caroline Gordon6, Sang-Cheol Bae7, Juanita Romero-Diaz8, Jorge Sanchez-Guerrero9, Sasha Bernatsky10, Daniel Wallace11, David Isenberg12, Anisur Rahman13, Joan Merrill14, Paul R Fortin15, Dafna Gladman16, Ian N. Bruce17, Michelle Petri18, Ellen M. Ginzler19, Mary Anne Dooley20, Rosalind Ramsey-Goldman21, Susan Manzi22, Andreas Jönsen23, Graciela Alarcón24, Ronald van Vollenhoven25, Cynthia Aranow26, Meggan Mackay26, Guillermo Ruiz-Irastorza27, S. Sam Lim28, Murat İnanç29, Kenneth Kalunian30, Soren Jacobsen31, Christine Peschken32, Diane Kamen33, Anca Askanase34, Jill Buyon35, Marvin Fritzler1, Ann E Clarke36 and May Choi37, 1University of Calgary, Calgary, AB, Canada, 2Division of Rheumatology, Queen Elizabeth II Health Sciences Center (Nova Scotia Rehabilitation Site) and Dalhousie University, Halifax, NS, Canada, 3University of Calgary, Ottawa, ON, Canada, 4University of Waterloo, Department of Geography & Environmental Management, Burlington, ON, Canada, 5University of Toronto, University Health Network, Schroeder Arthritis Institute, Toronto, ON, Canada, 6Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom, 7Hanyang University Medical Center, Seoul, Republic of Korea, 8Instituto Nacional de Ciencias Medicas y Nutricion SZ, Ciudad de México, Mexico, 9Mount Sinai Hospital and University Health Network, University of Toronto, Toronto, ON, Canada, 10Research Institute of the McGill University Health Centre, Montréal, QC, Canada, 11Cedars-Sinai Medical Center, Los Angeles, CA, 12University College London, London, United Kingdom, 13Centre for Rheumatology, Department of Medicine, University College London, London, United Kingdom, 14Oklahoma Medical Research Foundation, Oklahoma City, OK, 15Centre ARThrite - CHU de Québec - Université Laval, Québec, QC, Canada, 16Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 17Centre for Epidemiology Versus Arthritis, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom, 18Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 19SUNY Downstate Health Sciences University, Department of Medicine, Brooklyn, NY, 20Raleigh Neurology Associates, Chapel Hill, NC, 21Northwestern University Feinberg School of Medicine, Chicago, USA, Chicago, IL, 22Allegheny Health Network, Lupus Center of Excellence, Wexford, PA, 23Department of Clinical Sciences, Lund, Section for Rheumatology, Lund University, Lund and Skåne University Hospital, Lund, Sweden, 24The University of Alabama at Birmingham, Oakland, 25Amsterdam University Medical Centers, Amsterdam, Netherlands, 26Feinstein Institutes for Medical Research, Manhasset, NY, 27Autoimmune Diseases Research Unit, Biocruces Bizkaia Health Research Institute, Hospital Universitario Cruces, UPV/EHU, Barakaldo, Spain, 28Emory University, Atlanta, GA, 29Istanbul University Faculty of Medicine, Istanbul, Turkey, 30University of California San Diego, La Jolla, CA, 31Rigshospitalet, Copenhagen, Denmark, 32University of Manitoba, Winnipeg, MB, Canada, 33Medical University of South Carolina, Charleston, SC, 34Columbia University Medical Center, New York, NY, 35NYU Grossman School of Medicine, New York, NY, 36University of Calgary, Division of Rheumatology, Cumming School of Medicine, Calgary, AB, Canada, 37Brigham and Women's Hospital | University of Calgary, Calgary, AB, Canada
Background/Purpose: We previously reported in a single centre prevalent SLE cohort that antibodies against the cytokinesis-associated protein M-Phase Phosphoprotein 1 (anti-MPP-1) were associated with SLE-related cranial neuropathy (CN), a rare manifestation of neuropsychiatric SLE (NPSLE). The purpose of this study was to assess whether anti-MPP-1 is a biomarker for CN or other NPSLE manifestations using an international SLE inception cohort.
Methods: SLE patients fulfilling the updated 1997 ACR classification criteria for SLE were included. Anti-MPP-1 antibody testing was performed on baseline samples (within 15 months of diagnosis) or first annual assessment using an addressable laser bead immunoassay (ALBIA) with purified recombinant human protein with results expressed as median florescence units (MFU). Based on healthy controls, a dilution of ≥1:500 MFU was considered positive. NPSLE manifestations occurring over the first 5 years of follow up were documented annually based on ACR case definitions using published NPSLE attribution rules [1]). The frequency of anti-MPP-1 positivity between patients with versus without each of the 19 NPSLE manifestations was compared using univariate logistic regression. For any NPSLE manifestations where anti-MPP-1 positivity differed between patients with versus without the manifestation, baseline demographic and clinical characteristics were compared using T-tests and two-sample tests of proportions. For NPSLE manifestations associated with anti-MPP-1 positivity in the univariate analysis, multivariable logistic regression analysis using penalized maximum likelihood estimates was then performed to assess the relationship between anti-MPP-1 and the NPSLE manifestation, adjusting for age at anti-MPP-1 testing, female, White race/ethnicity, and significantly different baseline clinical characteristics.
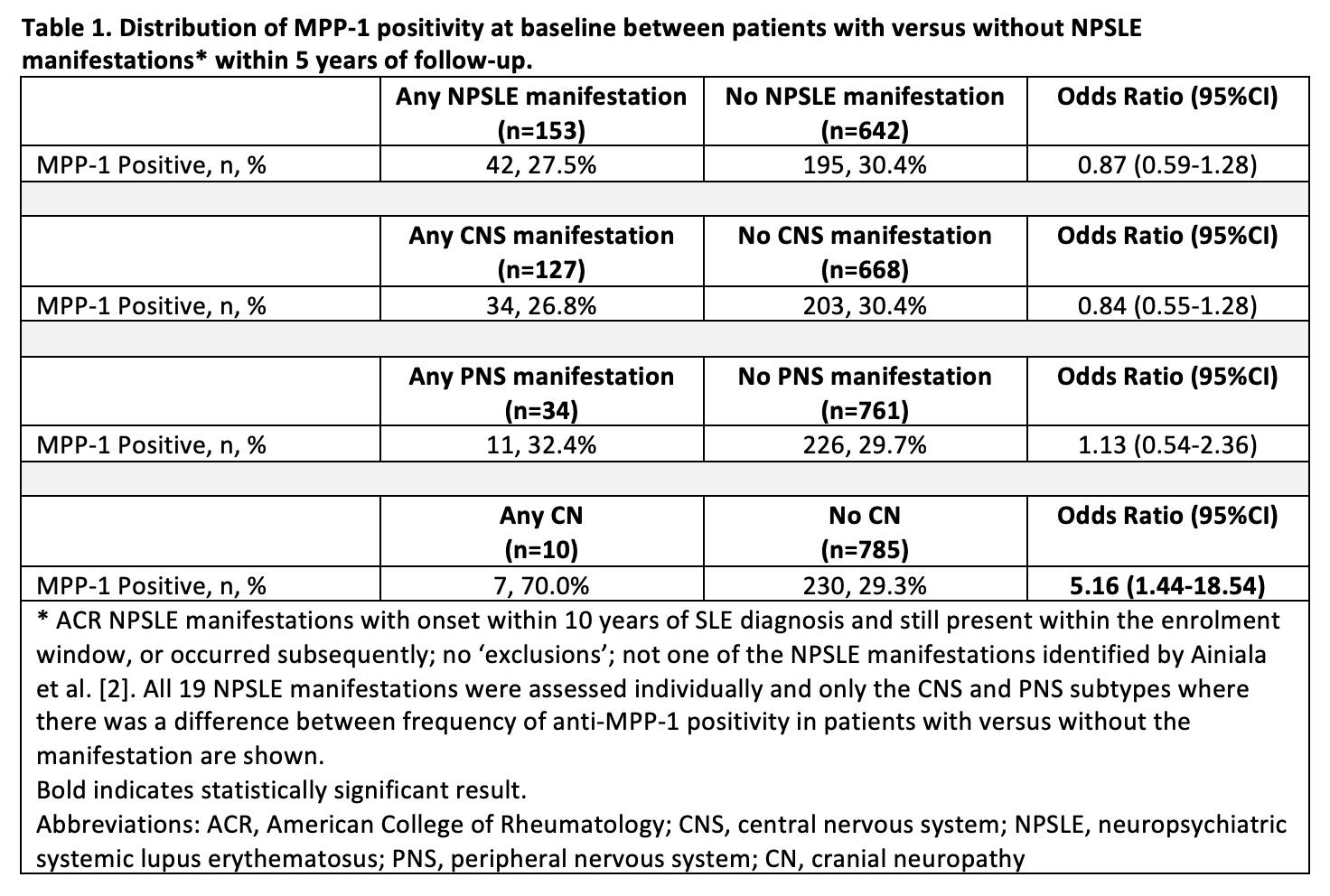
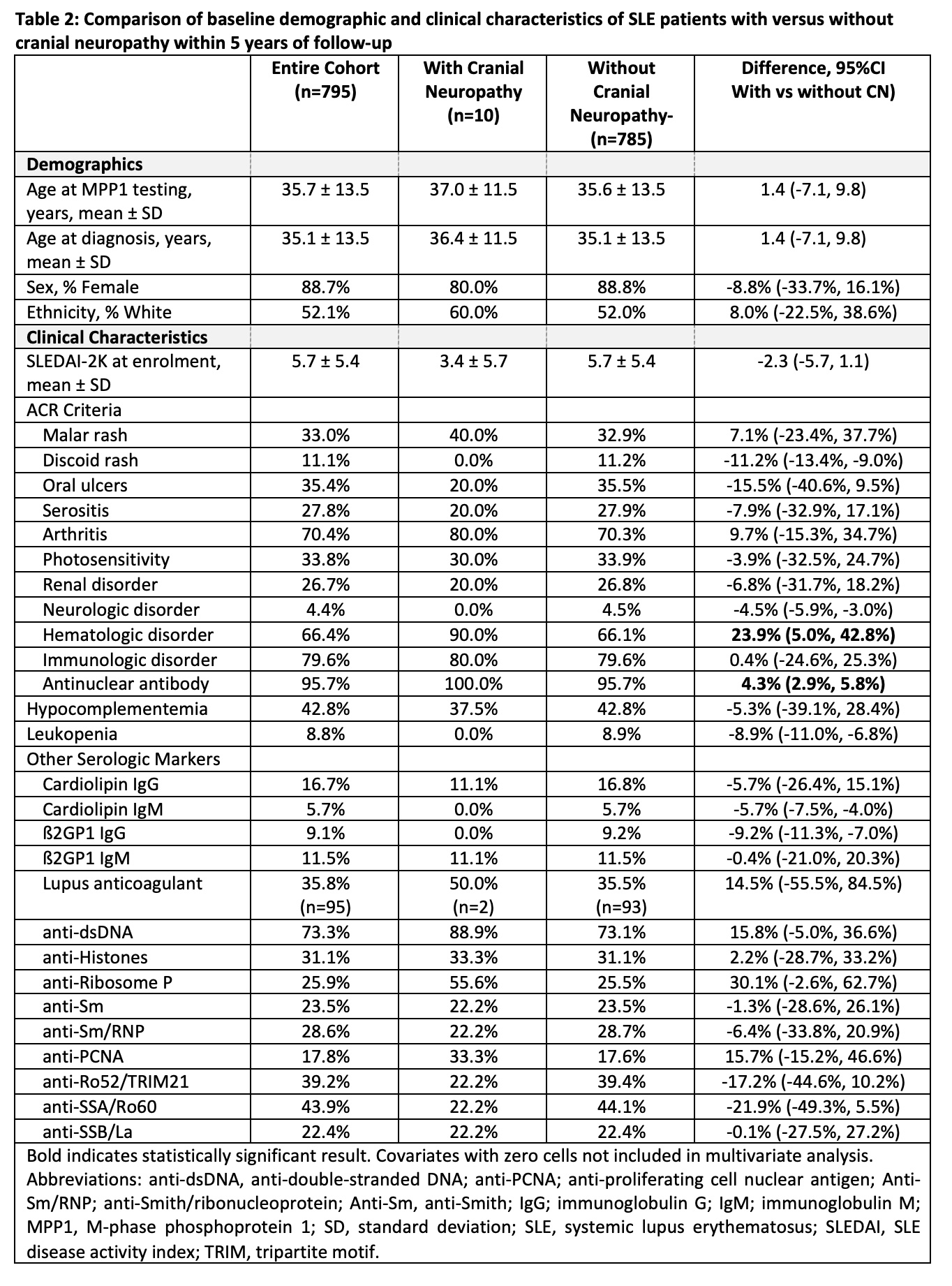
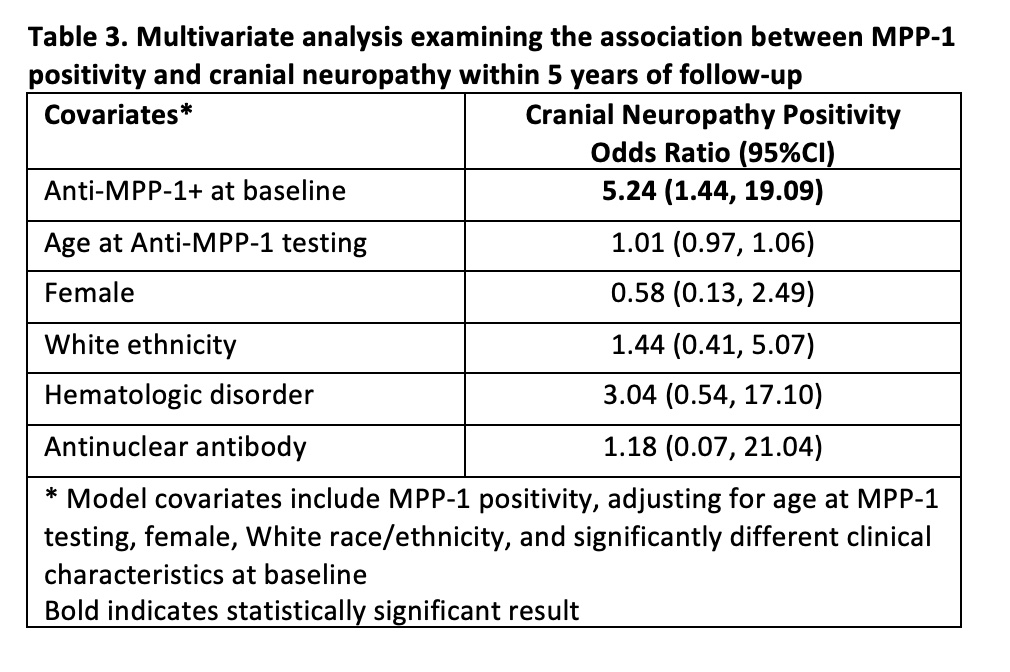
Results: Seven hundred and ninety-five SLE patients with complete data for 5 years of follow up were assessed; 29.8% were anti-MPP1 positive, 88.7% female, and 52.1% White. The frequency of anti-MPP-1 positivity differed only for those with versus without CN (70.0% vs. 29.3%; odds ratio [OR] 5.16, 95%CI 1.44, 18.54) (Table 1). Compared to patients without CN (n=785), patients with CN (n=10) were more likely to fulfill the ACR hematologic (difference: 23.9%, 95%CI 5.0%, 42.8%) and antinuclear antibody criteria (difference: 4.3%, 95%CI 2.9%, 5.8%) (Table 2). In the multivariate analysis, anti-MPP1 remained associated with CN (OR 5.24, 95%CI 1.44, 19.09) after adjusting for age at anti-MPP-1 testing, female, White race/ethnicity, hematologic disorder, and antinuclear antibody (Table 3).
Conclusion: Anti-MPP-1 is a potential biomarker for CN in SLE. Although anti-MPP-1 is differentially expressed in a variety of neurological cells and tissues, the link to a pathogenic role requires further study.
References:
1. Hanly, J. G., Urowitz, M. B., Gordon, C., et al. Ann Rheum Dis. 2020; 79(3): 356-362.
2. Ainiala H , Hietaharju A , Loukkola J , et al . Arthritis Rheum 2001 ;45: 419–23.
Disclosures: E. Krustev, Intercept Pharmaceuticals Inc, Mountain Valley MD Holdings INC, Gilead Sciences INC; J. Hanly, None; R. Chin, None; K. Buhler, None; F. Cardwell, None; M. Urowitz, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP; S. Bae, None; J. Romero-Diaz, Biogen; J. Sanchez-Guerrero, None; S. Bernatsky, None; D. Wallace, None; D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; A. Rahman, None; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; P. Fortin, AstraZeneca, GlaxoSmithKlein(GSK); D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; E. Ginzler, Aurinia Pharma; M. Dooley, None; R. Ramsey-Goldman, None; S. Manzi, AstraZeneca, GlaxoSmithKline (GSK), Exagen Diagnostics Inc, AbbVie, HGS, Cugene, Lilly, UCB Advisory Board, Lupus Foundation of America; A. Jönsen, None; G. Alarcón, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; C. Aranow, None; M. Mackay, None; G. Ruiz-Irastorza, None; S. Lim, None; M. İnanç, None; K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Jacobsen, None; C. Peschken, None; D. Kamen, None; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); J. Buyon, None; M. Fritzler, Mitogen Diagnostics Corporation; A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche.
Background/Purpose: We previously reported in a single centre prevalent SLE cohort that antibodies against the cytokinesis-associated protein M-Phase Phosphoprotein 1 (anti-MPP-1) were associated with SLE-related cranial neuropathy (CN), a rare manifestation of neuropsychiatric SLE (NPSLE). The purpose of this study was to assess whether anti-MPP-1 is a biomarker for CN or other NPSLE manifestations using an international SLE inception cohort.
Methods: SLE patients fulfilling the updated 1997 ACR classification criteria for SLE were included. Anti-MPP-1 antibody testing was performed on baseline samples (within 15 months of diagnosis) or first annual assessment using an addressable laser bead immunoassay (ALBIA) with purified recombinant human protein with results expressed as median florescence units (MFU). Based on healthy controls, a dilution of ≥1:500 MFU was considered positive. NPSLE manifestations occurring over the first 5 years of follow up were documented annually based on ACR case definitions using published NPSLE attribution rules [1]). The frequency of anti-MPP-1 positivity between patients with versus without each of the 19 NPSLE manifestations was compared using univariate logistic regression. For any NPSLE manifestations where anti-MPP-1 positivity differed between patients with versus without the manifestation, baseline demographic and clinical characteristics were compared using T-tests and two-sample tests of proportions. For NPSLE manifestations associated with anti-MPP-1 positivity in the univariate analysis, multivariable logistic regression analysis using penalized maximum likelihood estimates was then performed to assess the relationship between anti-MPP-1 and the NPSLE manifestation, adjusting for age at anti-MPP-1 testing, female, White race/ethnicity, and significantly different baseline clinical characteristics.
Results: Seven hundred and ninety-five SLE patients with complete data for 5 years of follow up were assessed; 29.8% were anti-MPP1 positive, 88.7% female, and 52.1% White. The frequency of anti-MPP-1 positivity differed only for those with versus without CN (70.0% vs. 29.3%; odds ratio [OR] 5.16, 95%CI 1.44, 18.54) (Table 1). Compared to patients without CN (n=785), patients with CN (n=10) were more likely to fulfill the ACR hematologic (difference: 23.9%, 95%CI 5.0%, 42.8%) and antinuclear antibody criteria (difference: 4.3%, 95%CI 2.9%, 5.8%) (Table 2). In the multivariate analysis, anti-MPP1 remained associated with CN (OR 5.24, 95%CI 1.44, 19.09) after adjusting for age at anti-MPP-1 testing, female, White race/ethnicity, hematologic disorder, and antinuclear antibody (Table 3).
Conclusion: Anti-MPP-1 is a potential biomarker for CN in SLE. Although anti-MPP-1 is differentially expressed in a variety of neurological cells and tissues, the link to a pathogenic role requires further study.
References:
1. Hanly, J. G., Urowitz, M. B., Gordon, C., et al. Ann Rheum Dis. 2020; 79(3): 356-362.
2. Ainiala H , Hietaharju A , Loukkola J , et al . Arthritis Rheum 2001 ;45: 419–23.



Disclosures: E. Krustev, Intercept Pharmaceuticals Inc, Mountain Valley MD Holdings INC, Gilead Sciences INC; J. Hanly, None; R. Chin, None; K. Buhler, None; F. Cardwell, None; M. Urowitz, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP; S. Bae, None; J. Romero-Diaz, Biogen; J. Sanchez-Guerrero, None; S. Bernatsky, None; D. Wallace, None; D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; A. Rahman, None; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; P. Fortin, AstraZeneca, GlaxoSmithKlein(GSK); D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; E. Ginzler, Aurinia Pharma; M. Dooley, None; R. Ramsey-Goldman, None; S. Manzi, AstraZeneca, GlaxoSmithKline (GSK), Exagen Diagnostics Inc, AbbVie, HGS, Cugene, Lilly, UCB Advisory Board, Lupus Foundation of America; A. Jönsen, None; G. Alarcón, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; C. Aranow, None; M. Mackay, None; G. Ruiz-Irastorza, None; S. Lim, None; M. İnanç, None; K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Jacobsen, None; C. Peschken, None; D. Kamen, None; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); J. Buyon, None; M. Fritzler, Mitogen Diagnostics Corporation; A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche.

