Back
Poster Session A
Osteoarthritis (OA) and related disorders
Session: (0017–0033) Osteoarthritis and Joint Biology – Basic Science Poster
0024: Exposure to Pollutants with Endocrine Disrupting Properties Is Associated with Early Cartilage Defects and Chondrocyte Inflammatory and Oxidative Activation
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SB
Sabryne Berkani, MS
Sorbonne université
Paris, France
Abstract Poster Presenter(s)
Sabryne Berkani1, Inés Kouki2, Sylvie Babajko3, Audrey Pigenet4, padmaja Natarajan5, Philip Ordoukhanian6, Xavier Houard4, Martin Lotz7, Barbara Demeneix8, Jean-Baptiste Fini8, Francis Berenbaum9, Jérémie SELLAM10 and Alice Courties11, 1Sorbonne Université, INSERM UMR 938, Centre de Recherche Saint-Antoine, Department of Rheumatology, Assistance Publique , Hôpital Saint-Antoine, Assistance Publique - Hôpitaux de Paris (AP-HP), Paris, France, Paris, 2Sorbonne Université, INSERM UMR 938, Centre de Recherche Saint-Antoine, Department of Rheumatology, Assistance Publique , Hôpital Saint-Antoine, Assistance Publique - Hôpitaux de Paris (AP-HP), Paris, France, Paris, France, 3Centre de Recherche des Cordeliers, INSERM, Sorbonne Université, Université de Paris, Paris, France, Paris, 4Sorbonne Université, INSERM UMR 938, Centre de Recherche Saint-Antoine, Hôpital Saint-Antoine, Assistance Publique - Hôpitaux de Paris (AP-HP), Paris, France, Paris, 5Center for Computational Biology & Bioinformatics and Genomics Core, Scripps Research, La Jolla, California, USA, La Jolla, 6Center for Computational Biology & Bioinformatics and Genomics Core, Scripps Research, La Jolla, CA, 7Scripps Research, La Jolla, 8UMR 7221, Phyma, CNRS-Muséum National d'Histoire Naturelle, Sorbonne Université, 75005 Paris, France., Paris, 9Sorbonne University - Saint-Antoine hospital, Paris, France, 10Sorbonne Universite, AP-HP, Saint-Antoine hospital, Paris, France, 11Service de Rhumatologie, AP-HP Hopital Saint-Antoine, Sorbonne Universite, INSERM, Centre de Recherche Saint-Antoine, Paris, France, Paris, France
Background/Purpose: Humans are exposed to pollutants with endocrine disruptors (ED) properties from their in utero life. Among them, a mix of 15 pollutants (perfluorinated compounds, bisphenol A, phthalates …) ubiquitously present (i.e, amniotic fluid of pregnant women, blood sera) has been associated with thyroid disruption and for some of them with language delay acquisition in children of exposed women (1). Since pollutants exposure has been associated with an increased risk of joint diseases (2), we aimed to determine the effects of the pollutants mix on cartilage homeostasis and chondrocyte function.
Methods: Female C57/B6 mice were exposed to a mix of 15 pollutants in their drinking water (equivalent dose of perfluorooctanoic acid [PFOA] 0.1 µg/day) 15 days prior to mating and during gestation. The newborn mice of exposed mothers were also exposed to the same mix until their sacrifice at 10 months (n= 18). A parallel group of unexposed female mice and newborn mice served as controls (n=18). At sacrifice, knees were harvested and we analyzed histological analysis of cartilage degradation, synovium inflammation and subchondral bone areas (Bone area/Total area ratio). In vitro, primary culture of articular chondrocytes isolated from newborn C57/B6 mice (n=7) were exposed or not to different doses of the mix of pollutants for 24h. We evaluated cell toxicity by LDH, the expression of cytokines (interleukin [IL] -6) chemokines (MCP1) and metalloproteases (MMP-3) by RT-qPCR and ELISA. After 15 minutes of exposure, we also evaluated the MAPK38 phosphorylation by Western Blot. Finally, we determined the differentially expressed (DE) genes by whole genome RNA-sequencing between murine chondrocytes treated or not by 24 h of mix (eq PFOA 0.03 µg/mL) to identify pathways involved in cell activation.
Results: At 10 months, the mean OARSI score was higher in exposed male than in controlled male (3.25 + 0.31 vs 2.12 + 0.31 p=0.02), but not in female (Fig.1). There was no difference in mouse phenotype (height, weight, behavior), subchondral bone areas nor in synovitis. In vitro, the mix of pollutants did not induce a direct cell toxicity (LDH) while it increased the expression of inflammatory cytokines and metalloproteinases by qPCR [IL6 : + 222% (p< 0.05) ; MCP 1 : + 155% (p< 0.05) , MMP-3 : +222% (p< 0.05) for the mix at eq. PFOA 0.03 µg/mL] with a dose-effect (Fig. 2). Similar results were observed in ELISA. At 15 minutes, the mix (eq PFOA 0.03 µg/mL) significantly increased p38 MAPK phosphorylation. Among the 256 DE genes, 214 were upregulated and 42 downregulated in exposed chondrocytes. The gene ontology identified several pathways involved in oxidative stress such as glutathione metabolism, pentose phosphate pathway and ferroptosis (Fig. 3)
Conclusion: ED exposition induces early cartilage defects in mice that could be related to a local increased secretion of inflammatory cytokines and metalloproteinases by chondrocytes and to chondrocytic oxidative stress enhancement. Altogether, this study suggests that exposition to pollutants disturbs cartilage homeostasis, which can increase susceptibility to joint diseases.
.jpg) Cartilage OARSI score (0-12) of 10 months mice unexposed or exposed to the mix of 15 pollutants (equivalent dose of perfluorooctanoic acid [PFOA] 0.1 µg/day) from their in utero life, *p-value < 0.05 with non-parametric test.
Cartilage OARSI score (0-12) of 10 months mice unexposed or exposed to the mix of 15 pollutants (equivalent dose of perfluorooctanoic acid [PFOA] 0.1 µg/day) from their in utero life, *p-value < 0.05 with non-parametric test.
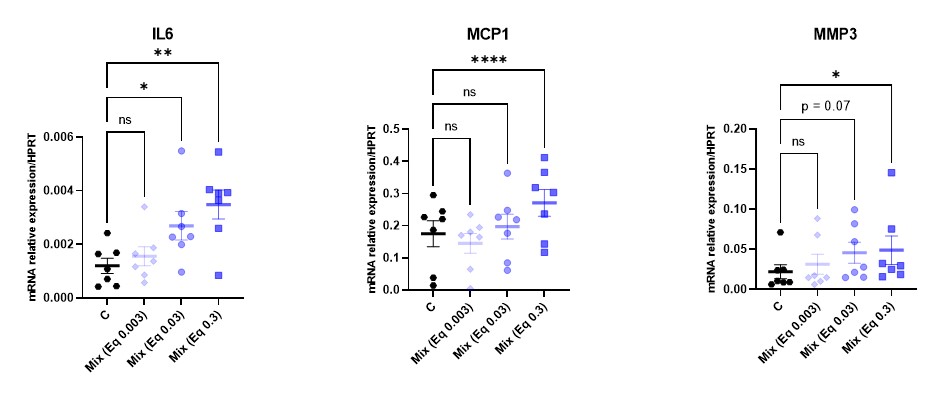 mRNA expression of IL6, MCP1 and MMP3 in unexposed (C for control) and exposed murine chondrocytes for 24 h by different dose of the mix of pollutants mix (equivalent dose of PFOA µg/mL), *p-value < 0.05; **p-value < 0.01, ****p-value < 0.0001 using one-way ANOVA with Dunnett post-test.
mRNA expression of IL6, MCP1 and MMP3 in unexposed (C for control) and exposed murine chondrocytes for 24 h by different dose of the mix of pollutants mix (equivalent dose of PFOA µg/mL), *p-value < 0.05; **p-value < 0.01, ****p-value < 0.0001 using one-way ANOVA with Dunnett post-test.
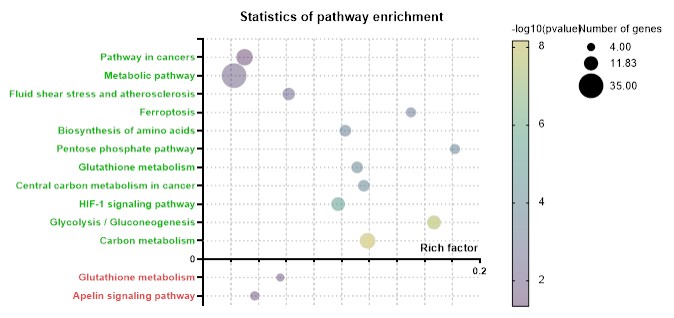 Statistics of pathway enrichment of whole genome sequencing of murine chondrocytes exposed or no 24 h to the mix of 15 pollutants, the green pathways are the pathways involving the DE upregulated genes while the red are the pathways downregulated in exposed chondrocytes. . The rich factor is the ratio of the number of involved genes in the pathway found in our transcriptomic analysis (size of the bubble) on the total number of the known genes of the pathway.
Statistics of pathway enrichment of whole genome sequencing of murine chondrocytes exposed or no 24 h to the mix of 15 pollutants, the green pathways are the pathways involving the DE upregulated genes while the red are the pathways downregulated in exposed chondrocytes. . The rich factor is the ratio of the number of involved genes in the pathway found in our transcriptomic analysis (size of the bubble) on the total number of the known genes of the pathway.
Disclosures: S. Berkani, None; I. Kouki, None; S. Babajko, None; A. Pigenet, None; p. Natarajan, None; P. Ordoukhanian, None; X. Houard, None; M. Lotz, None; B. Demeneix, None; J. Fini, None; F. Berenbaum, 4Moving Biotech; J. SELLAM, None; A. Courties, None.
Background/Purpose: Humans are exposed to pollutants with endocrine disruptors (ED) properties from their in utero life. Among them, a mix of 15 pollutants (perfluorinated compounds, bisphenol A, phthalates …) ubiquitously present (i.e, amniotic fluid of pregnant women, blood sera) has been associated with thyroid disruption and for some of them with language delay acquisition in children of exposed women (1). Since pollutants exposure has been associated with an increased risk of joint diseases (2), we aimed to determine the effects of the pollutants mix on cartilage homeostasis and chondrocyte function.
Methods: Female C57/B6 mice were exposed to a mix of 15 pollutants in their drinking water (equivalent dose of perfluorooctanoic acid [PFOA] 0.1 µg/day) 15 days prior to mating and during gestation. The newborn mice of exposed mothers were also exposed to the same mix until their sacrifice at 10 months (n= 18). A parallel group of unexposed female mice and newborn mice served as controls (n=18). At sacrifice, knees were harvested and we analyzed histological analysis of cartilage degradation, synovium inflammation and subchondral bone areas (Bone area/Total area ratio). In vitro, primary culture of articular chondrocytes isolated from newborn C57/B6 mice (n=7) were exposed or not to different doses of the mix of pollutants for 24h. We evaluated cell toxicity by LDH, the expression of cytokines (interleukin [IL] -6) chemokines (MCP1) and metalloproteases (MMP-3) by RT-qPCR and ELISA. After 15 minutes of exposure, we also evaluated the MAPK38 phosphorylation by Western Blot. Finally, we determined the differentially expressed (DE) genes by whole genome RNA-sequencing between murine chondrocytes treated or not by 24 h of mix (eq PFOA 0.03 µg/mL) to identify pathways involved in cell activation.
Results: At 10 months, the mean OARSI score was higher in exposed male than in controlled male (3.25 + 0.31 vs 2.12 + 0.31 p=0.02), but not in female (Fig.1). There was no difference in mouse phenotype (height, weight, behavior), subchondral bone areas nor in synovitis. In vitro, the mix of pollutants did not induce a direct cell toxicity (LDH) while it increased the expression of inflammatory cytokines and metalloproteinases by qPCR [IL6 : + 222% (p< 0.05) ; MCP 1 : + 155% (p< 0.05) , MMP-3 : +222% (p< 0.05) for the mix at eq. PFOA 0.03 µg/mL] with a dose-effect (Fig. 2). Similar results were observed in ELISA. At 15 minutes, the mix (eq PFOA 0.03 µg/mL) significantly increased p38 MAPK phosphorylation. Among the 256 DE genes, 214 were upregulated and 42 downregulated in exposed chondrocytes. The gene ontology identified several pathways involved in oxidative stress such as glutathione metabolism, pentose phosphate pathway and ferroptosis (Fig. 3)
Conclusion: ED exposition induces early cartilage defects in mice that could be related to a local increased secretion of inflammatory cytokines and metalloproteinases by chondrocytes and to chondrocytic oxidative stress enhancement. Altogether, this study suggests that exposition to pollutants disturbs cartilage homeostasis, which can increase susceptibility to joint diseases.
.jpg) Cartilage OARSI score (0-12) of 10 months mice unexposed or exposed to the mix of 15 pollutants (equivalent dose of perfluorooctanoic acid [PFOA] 0.1 µg/day) from their in utero life, *p-value < 0.05 with non-parametric test.
Cartilage OARSI score (0-12) of 10 months mice unexposed or exposed to the mix of 15 pollutants (equivalent dose of perfluorooctanoic acid [PFOA] 0.1 µg/day) from their in utero life, *p-value < 0.05 with non-parametric test.  mRNA expression of IL6, MCP1 and MMP3 in unexposed (C for control) and exposed murine chondrocytes for 24 h by different dose of the mix of pollutants mix (equivalent dose of PFOA µg/mL), *p-value < 0.05; **p-value < 0.01, ****p-value < 0.0001 using one-way ANOVA with Dunnett post-test.
mRNA expression of IL6, MCP1 and MMP3 in unexposed (C for control) and exposed murine chondrocytes for 24 h by different dose of the mix of pollutants mix (equivalent dose of PFOA µg/mL), *p-value < 0.05; **p-value < 0.01, ****p-value < 0.0001 using one-way ANOVA with Dunnett post-test.  Statistics of pathway enrichment of whole genome sequencing of murine chondrocytes exposed or no 24 h to the mix of 15 pollutants, the green pathways are the pathways involving the DE upregulated genes while the red are the pathways downregulated in exposed chondrocytes. . The rich factor is the ratio of the number of involved genes in the pathway found in our transcriptomic analysis (size of the bubble) on the total number of the known genes of the pathway.
Statistics of pathway enrichment of whole genome sequencing of murine chondrocytes exposed or no 24 h to the mix of 15 pollutants, the green pathways are the pathways involving the DE upregulated genes while the red are the pathways downregulated in exposed chondrocytes. . The rich factor is the ratio of the number of involved genes in the pathway found in our transcriptomic analysis (size of the bubble) on the total number of the known genes of the pathway. Disclosures: S. Berkani, None; I. Kouki, None; S. Babajko, None; A. Pigenet, None; p. Natarajan, None; P. Ordoukhanian, None; X. Houard, None; M. Lotz, None; B. Demeneix, None; J. Fini, None; F. Berenbaum, 4Moving Biotech; J. SELLAM, None; A. Courties, None.

