Back
Ignite Talk
Session: Ignite Session 8C
L12: First Line Treatment Using Recombinant IL-1Receptor Antagonist in New Onset Systemic Juvenile Idiopathic Arthritis Is an Effective Treatment Strategy, Irrespective of HLA DRB1 Background
Monday, November 14, 2022
2:25 PM – 2:30 PM Eastern Time
Location: South Philly Stage
- SV
Sebastiaan Vastert, MD, PhD
University Medical Center Utrecht
Utrecht, NetherlandsDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Remco Erkens1, Rashmi Sinha2, Alex Pickering3, Grant Schulert4, Alexei Grom4, Lars van der Veken1, Hanneke van Deutekom1, Jorg Calis1, Jorg van Loosdregt5 and Sebastiaan Vastert1, 1University Medical Center Utrecht, Utrecht, Netherlands, 2Systemic JIA Foundation, Cincinnati, OH, 3Systemic JIA Foundation, San Francisso, CA, 4Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 5University Medical Center Utrecht, Wilhelmina Children's Hospital, Zeist, Netherlands
Background/Purpose: Systemic Juvenile Idiopathic Arthritis (sJIA) is a severe subtype of JIA. Recently, interstitial lung disease (SJIA-LD) has been reported as a severe complication of sJIA, specifically in the first 2 years of disease. Suggested risk factors for the development of sJIA-ILD are disease onset at young age, the occurrence of macrophage activation syndrome (MAS), and adverse drug reactions to biologicals (both IL-1 and IL-6). Adverse drug reactions have recently been associated to HLA DRB1*15 haplotypes. This finding has spurred a debate among pediatric rheumatologist as concerns the use of advises on pre-prescription HLA-typing to guide medication decisions for new onset sJIA patients. Such decisions may include postponing and even forgoing the initiation of effective biological therapy in new onset sJIA patients with HLA-DRB1*15 haplotypes.
Methods: HLA DRB genotyping by whole genome sequencing.
Prospective and standardized treatment cohort study, all started with recombinant IL-1Receptor antagonist (anakinra) as 1st-line therapy/biological.
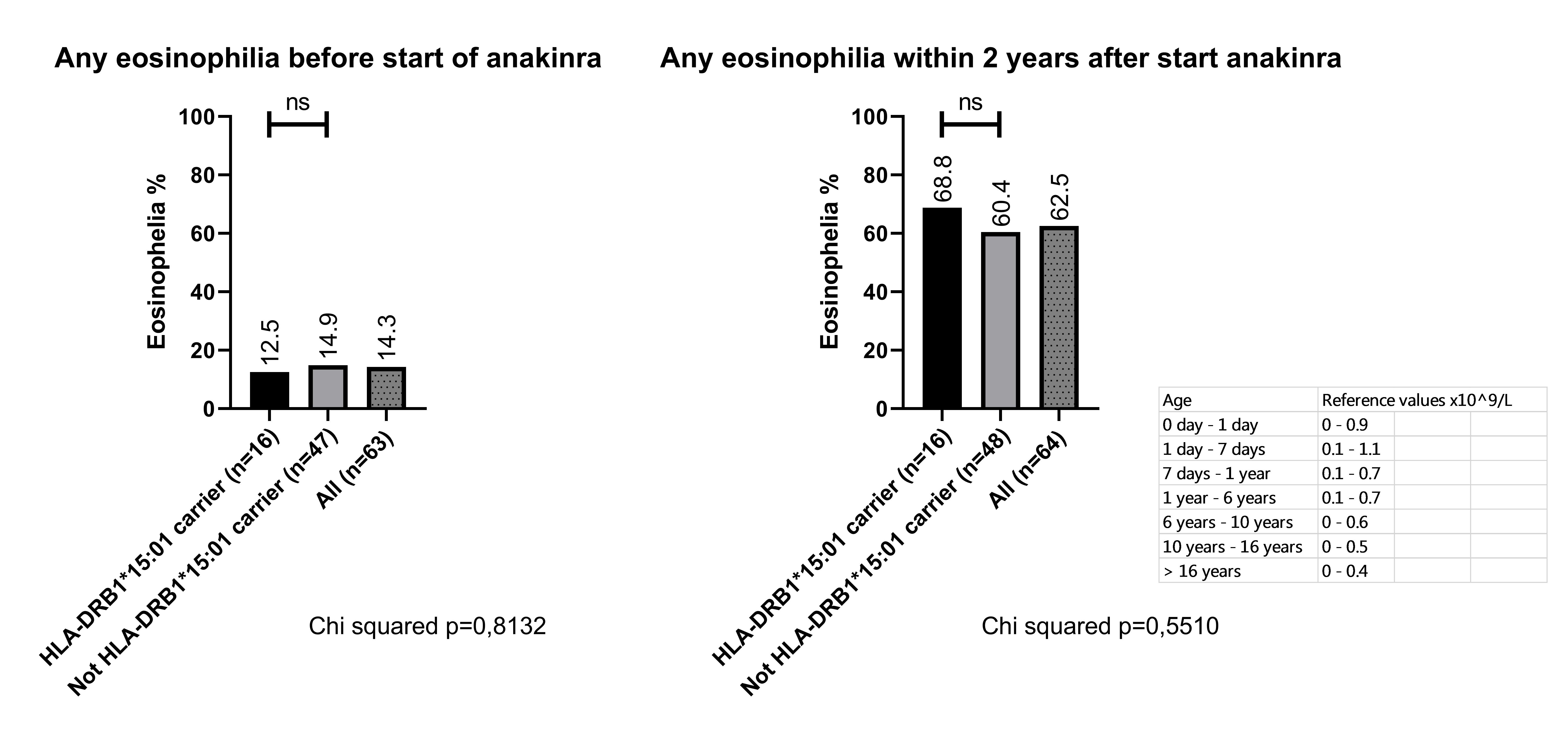
Results: Our prospective cohort consists of 65 SJIA patients, 53,9% male, mean age at diagnosis of 7.4 years old (range 0.8 to 15.7 yrs). Anakinra was started as 1st line therapy in 60/65 patients, and as 1st line biological in 5/65 (after corticosteroid therapy before referral). In total, 72 % of patients reached a steroid-free inactive disease (complete response) at 6 months and 68% after 1 year. In the first 2 years of disease, 20% of patients developed MAS and 62.5% developed eosinophilia at least at 1 time point (≥ reference values adjusted for age), of which 9/65 (13,8%) patients at time of diagnosis. We found that 17/65 patients (26,1%) carry 1 HLA-DRB1*15:01:01 allele and 21/65 (32,3%) carry HLA DRB1.11 (known risk locus for sJIA). The rate of HLA-DRB1*15:01 in our cohort is comparable to the general European and US population. Of the 17 patients with HLA-DRB1*15:01, one patient developed SJIA-LD during follow-up of at least 2 years. Importantly, the response rate to anakinra in our cohort did not differ significantly between carriers of HLA DRB1*15:01 and non-carriers (figure 1, complete response at 6 months 59% vs 77% p=0.15, complete response at 1 year 52,9% vs 72,9% p=0.13, Chi2). In addition, we observed no significant difference in IL-18 levels at the start of treatment, nor in the occurrence of eosinophilia in the first 2 years between HLA-DRB1*15:01 and non-carriers (figure 2). Two patients had to discontinue anakinra due to adverse reactions to the drug, but both appeared not to be carriers of HLA-DRB1*15:01.
Conclusion: Our cohort showed an HLA DRB1*15:01 carriage of 17/65 (26,1%), comparable to the general population. Although one patient in our cohort developed interstitial lung disease and was a carrier of HLA DRB1*15:01, we did not see differences in the occurrence of eosinophilia and response to anakinra in the first 2 years of disease between HLA DRB1.15 carriers or non-carriers. We suggest not to withhold effective biological therapy in new sJIA patients based on HLA type and only consider (switching to) alternative glucocorticoid based treatment regimens in patients that do not achieve inactive disease.
.jpg)
Disclosures: R. Erkens, None; R. Sinha, None; A. Pickering, None; G. Schulert, Novartis, Sobi, AB2 Bio, NovImmune; A. Grom, Novartis, Sobi, AB2Bio; L. van der Veken, None; H. van Deutekom, None; J. Calis, None; J. van Loosdregt, None; S. Vastert, Sobi, Novartis.
Background/Purpose: Systemic Juvenile Idiopathic Arthritis (sJIA) is a severe subtype of JIA. Recently, interstitial lung disease (SJIA-LD) has been reported as a severe complication of sJIA, specifically in the first 2 years of disease. Suggested risk factors for the development of sJIA-ILD are disease onset at young age, the occurrence of macrophage activation syndrome (MAS), and adverse drug reactions to biologicals (both IL-1 and IL-6). Adverse drug reactions have recently been associated to HLA DRB1*15 haplotypes. This finding has spurred a debate among pediatric rheumatologist as concerns the use of advises on pre-prescription HLA-typing to guide medication decisions for new onset sJIA patients. Such decisions may include postponing and even forgoing the initiation of effective biological therapy in new onset sJIA patients with HLA-DRB1*15 haplotypes.
Methods: HLA DRB genotyping by whole genome sequencing.
Prospective and standardized treatment cohort study, all started with recombinant IL-1Receptor antagonist (anakinra) as 1st-line therapy/biological.
Results: Our prospective cohort consists of 65 SJIA patients, 53,9% male, mean age at diagnosis of 7.4 years old (range 0.8 to 15.7 yrs). Anakinra was started as 1st line therapy in 60/65 patients, and as 1st line biological in 5/65 (after corticosteroid therapy before referral). In total, 72 % of patients reached a steroid-free inactive disease (complete response) at 6 months and 68% after 1 year. In the first 2 years of disease, 20% of patients developed MAS and 62.5% developed eosinophilia at least at 1 time point (≥ reference values adjusted for age), of which 9/65 (13,8%) patients at time of diagnosis. We found that 17/65 patients (26,1%) carry 1 HLA-DRB1*15:01:01 allele and 21/65 (32,3%) carry HLA DRB1.11 (known risk locus for sJIA). The rate of HLA-DRB1*15:01 in our cohort is comparable to the general European and US population. Of the 17 patients with HLA-DRB1*15:01, one patient developed SJIA-LD during follow-up of at least 2 years. Importantly, the response rate to anakinra in our cohort did not differ significantly between carriers of HLA DRB1*15:01 and non-carriers (figure 1, complete response at 6 months 59% vs 77% p=0.15, complete response at 1 year 52,9% vs 72,9% p=0.13, Chi2). In addition, we observed no significant difference in IL-18 levels at the start of treatment, nor in the occurrence of eosinophilia in the first 2 years between HLA-DRB1*15:01 and non-carriers (figure 2). Two patients had to discontinue anakinra due to adverse reactions to the drug, but both appeared not to be carriers of HLA-DRB1*15:01.
Conclusion: Our cohort showed an HLA DRB1*15:01 carriage of 17/65 (26,1%), comparable to the general population. Although one patient in our cohort developed interstitial lung disease and was a carrier of HLA DRB1*15:01, we did not see differences in the occurrence of eosinophilia and response to anakinra in the first 2 years of disease between HLA DRB1.15 carriers or non-carriers. We suggest not to withhold effective biological therapy in new sJIA patients based on HLA type and only consider (switching to) alternative glucocorticoid based treatment regimens in patients that do not achieve inactive disease.
.jpg)
Complete response (clinically inactive disease withouth glucocorticoids) rates stratified to HLA DRB1.15:01 carriage or not.

Prevalence of peripheral blood eosinophilia stratified to HLA DRB1.15:01 carriage or not.
Disclosures: R. Erkens, None; R. Sinha, None; A. Pickering, None; G. Schulert, Novartis, Sobi, AB2 Bio, NovImmune; A. Grom, Novartis, Sobi, AB2Bio; L. van der Veken, None; H. van Deutekom, None; J. Calis, None; J. van Loosdregt, None; S. Vastert, Sobi, Novartis.

