Back
Late-Breaking Abstract Session
Session: Late-Breaking Abstracts (L01–L06)
L06: Risk of Extended Major Adverse Cardiovascular Event Endpoints with Tofacitinib vs TNF Inhibitors in Patients with Rheumatoid Arthritis: A Post Hoc Analysis of a Phase 3b/4 Randomized Safety Study
Monday, November 14, 2022
10:15 AM – 10:25 AM Eastern Time
Location: Exhibit Hall A
.png)
Maya Buch, MD, PhD
University of Manchester
Manchester, United Kingdom
Presenting Author(s)
Maya H. Buch1, Deepak L. Bhatt2, Christina Charles-Schoeman3, Jon T. Giles4, Ted R. Mikuls5, Gary Koch6, Steven Ytterberg7, Edward Nagy8, Hyejin Jo9, Kenneth Kwok9, Carol A. Connell10, Karim R. Masri11 and Arne Yndestad12, 1Centre for Musculoskeletal Research, Division of Musculoskeletal and Dermatological Sciences, Faculty of Biology, Medicine and Health, University of Manchester, and NIHR Manchester Biomedical Research Centre, Manchester, United Kingdom, 2Division of Cardiovascular Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, 3Division of Rheumatology, Department of Medicine, University of California, Los Angeles, CA, 4Division of Rheumatology, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, 5Division of Rheumatology, University of Nebraska Medical Center and VA Nebraska-Western Iowa Health Care System, Omaha, NE, 6Department of Biostatistics, University of North Carolina at Chapel Hill, Chapel Hill, NC, 7Division of Rheumatology, Mayo Clinic, Rochester, MN, 8Pfizer Ltd, Tadworth, Surrey, United Kingdom, 9Pfizer Inc, New York, NY, 10Pfizer Inc, Groton, CT, 11Pfizer Inc, Collegeville, PA, 12Pfizer Inc, Oslo, Norway
Background/Purpose: ORAL Surveillance (NCT02092467; a post-authorization safety study of tofacitinib 5 and 10 mg twice daily [BID] vs TNF inhibitors [TNFi]) found higher risk of major adverse cardiovascular (CV) events (MACE) and venous thromboembolism (VTE) with tofacitinib vs TNFi.1 A post hoc analysis of ORAL Surveillance found higher risk of MACE with tofacitinib vs TNFi in patients (pts) with history of atherosclerotic CV disease (ASCVD); risk did not appear different with tofacitinib 5 mg BID vs TNFi in pts without history of ASCVD.2 This post hoc analysis expands on 3-point MACE (MACE-3; a composite of CV death, and non-fatal MI and stroke) by evaluating risk of all adjudicated CV events as part of extended MACE endpoints in ORAL Surveillance with tofacitinib vs TNFi.
Methods: Pts with RA aged ≥50 years and with ≥1 additional CV risk factor received tofacitinib 5 mg (N=1,455) or 10 mg (N=1,456) BID, or TNFi (N=1,451). Extended MACE endpoints were based on MACE-3 and sequentially added adjudicated ischemic CV events (ie hospitalization for unstable angina [MACE-4], coronary revascularization procedures [MACE-5], transient ischemic attack [MACE-6], and peripheral vascular disease [MACE-7]), hospitalization for heart failure (HF; MACE-8), and VTE (MACE-8 plus VTE). Hazard ratios (HRs; time to first event analysis) were evaluated with tofacitinib vs TNFi for extended MACE endpoints (risk period up to first event of aggregated CV events) and for individual component endpoints (risk period up to first event of individual CV events), separately. Subgroup analyses by history of ASCVD were performed.
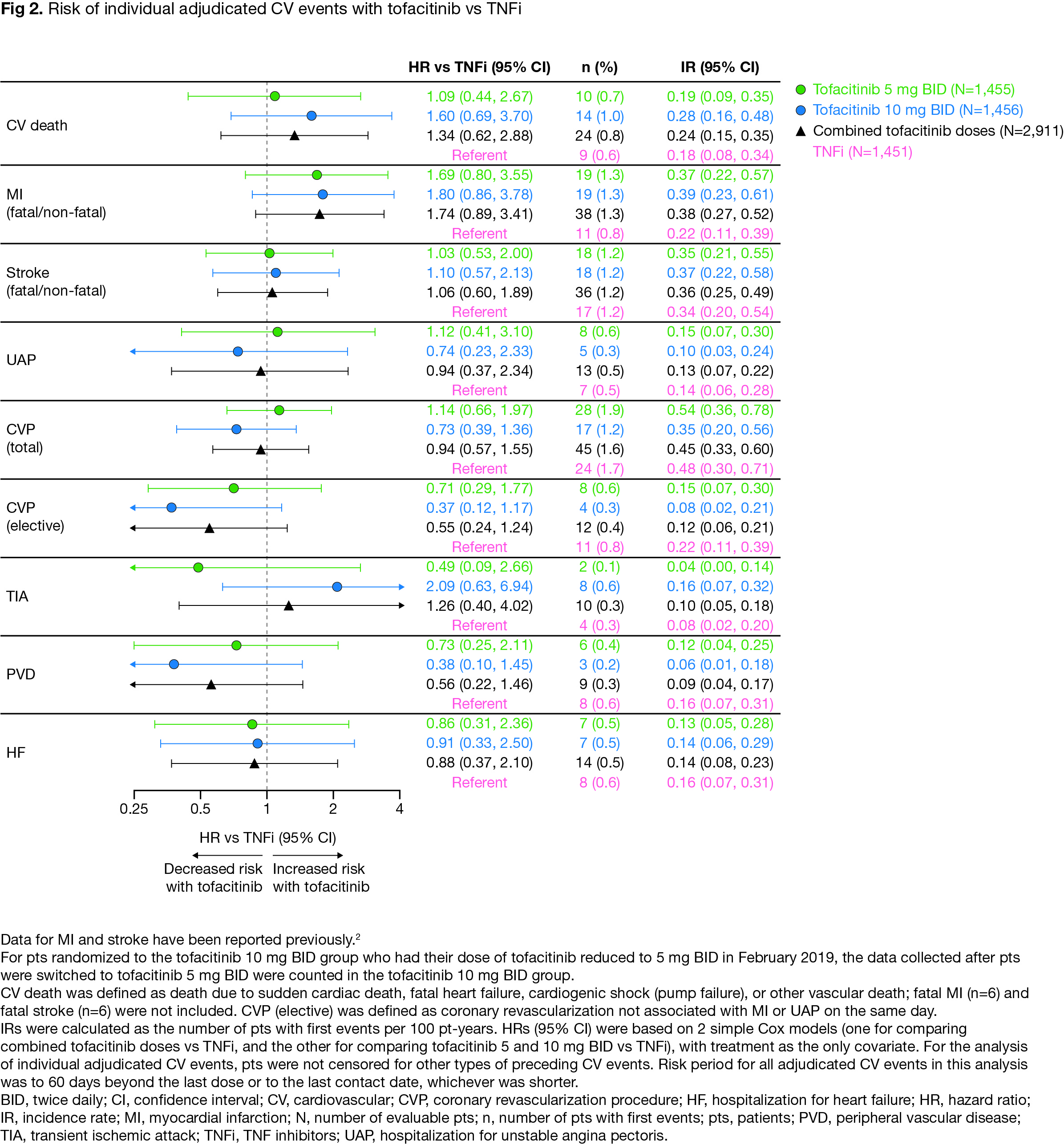
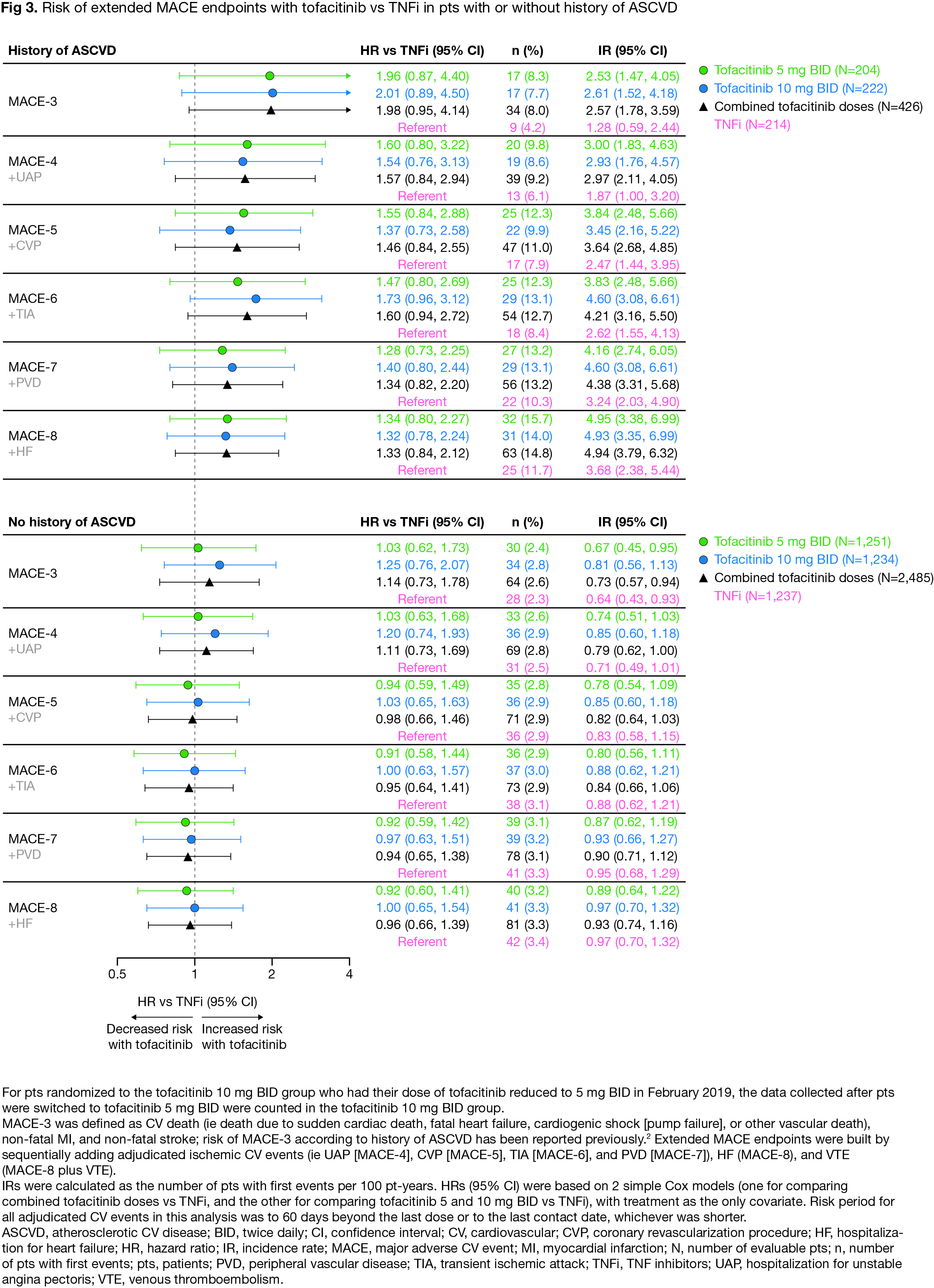
Results: For extended endpoints of adjudicated ischemic CV events (ie MACE-4 to -7), HRs with tofacitinib vs TNFi were similar to HRs for MACE-3 (Fig 1). Risk of MACE-8 was similar with combined tofacitinib doses vs TNFi (HR [95% confidence intervals (CI)] 1.08 [0.81, 1.44]). Risk of MACE-8 plus VTE was similar with tofacitinib 5 mg BID vs TNFi (HR 1.12 [0.82, 1.52]), but higher with tofacitinib 10 mg BID vs TNFi (HR 1.38 [1.02, 1.85]) (Fig 1). Risk of MI appeared higher with tofacitinib vs TNFi (HR 1.74 [0.89, 3.41], combined doses), but risk of other individual adjudicated CV events was generally similar (Fig 2). Across extended MACE definitions (ie up to MACE-8), risk appeared higher with tofacitinib vs TNFi in pts with history of ASCVD (Fig 3).
Conclusion: In ORAL Surveillance, risk of a composite of all ischemic CV events and HF (ie MACE-8) did not appear different with tofacitinib vs TNFi. However, across extended MACE endpoints risk was numerically higher with tofacitinib vs TNFi in pts with a history of ASCVD. The totality of CV risk (ie MACE-8 plus VTE) was higher with tofacitinib 10 mg BID vs TNFi, driven by an increase in VTE events. Limitations include low numbers of individual CV events and not considering severity/frequency of multiple events. These data highlight the need for a better understanding of risk of individual CV events in pts with RA.
Ytterberg et al. N Engl J Med 2022; 386: 316–26
Charles-Schoeman et al. Ann Rheum Dis 2022: ard-2022-222259. Epub ahead of print
Study sponsored by Pfizer. Medical writing support was provided by L Hogarth, CMC Connect, and funded by Pfizer.
.jpg)
Disclosures: M. Buch, AbbVie, Eli Lilly, Gilead Sciences, Pfizer Inc, UCB; D. Bhatt, Angio Wave, Bayer, Boehringer-Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, Myokardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences and Stasys, Bristol-Myers Squibb, High Enroll, Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Beren, Boston Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Javelin, Lexicon, Eli Lilly, Medtronic, Merck, Moderna, Myokardia, Novartis, Owkin, Pfizer Inc, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi-Regeneron, Stasys, Synaptic, The Medicines Company and 89bio, AngioWave (stock options), Boston VA Research Institute, DRS.LINQ (stock options), The Society of Cardiovascular Patient Care, TobeSoft, American Heart Association Quality Oversight Committee, Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute), The PORTICO trial, (funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, The Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), The ABILITY-DM trial (funded by Concept Medical), The Population Health Research Institute, Rutgers University (for the NIH-funded MINT trial), Deputy Editor for Clinical Cardiology, Trustee for the American College of Cardiology, American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Belvoir Publications (Editor in Chief, Harvard Heart Letter), The Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), The Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute, Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), The Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee), Elsevier (Editor, Braunwald’s Heart Disease), Named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Phillips, SpectraWAVE, Svelte, Vascular Solutions, Conducts unfunded research with FlowCo and Takeda; C. Charles-Schoeman, AbbVie, Gilead Sciences, Pfizer Inc, Sanofi-Regeneron, Bristol-Myers Squibb; J. Giles, AbbVie, Bristol-Myers Squibb, Eli Lilly, Genentech, Gilead Sciences, UCB, Pfizer Inc; T. Mikuls, Gilead Sciences, Horizon, Sanofi-Regeneron, Pfizer Inc, Bristol-Myers Squibb; G. Koch, University of North Carolina at Chapel Hill, AbbVie, Acceleron, Amgen, Arena, AstraZeneca, Cytokinetics, Eli Lilly, Gilead Sciences, GlaxoSmithKlein, Huya Bioscience International, Johnson & Johnson, Landos Biopharma, Merck, Momentum, Novartis, Otsuka, Pfizer Inc, Sanofi-Regeneron, vTv Therapeutics, IQVIA; S. Ytterberg, Corbus Pharmaceuticals, Janssen, Kezar Life Sciences, Pfizer Inc; E. Nagy, Pfizer Ltd; H. Jo, Syneos Health, Pfizer Inc; K. Kwok, Pfizer Inc; C. Connell, Pfizer Inc; K. Masri, Pfizer Inc; A. Yndestad, Pfizer Inc.
Background/Purpose: ORAL Surveillance (NCT02092467; a post-authorization safety study of tofacitinib 5 and 10 mg twice daily [BID] vs TNF inhibitors [TNFi]) found higher risk of major adverse cardiovascular (CV) events (MACE) and venous thromboembolism (VTE) with tofacitinib vs TNFi.1 A post hoc analysis of ORAL Surveillance found higher risk of MACE with tofacitinib vs TNFi in patients (pts) with history of atherosclerotic CV disease (ASCVD); risk did not appear different with tofacitinib 5 mg BID vs TNFi in pts without history of ASCVD.2 This post hoc analysis expands on 3-point MACE (MACE-3; a composite of CV death, and non-fatal MI and stroke) by evaluating risk of all adjudicated CV events as part of extended MACE endpoints in ORAL Surveillance with tofacitinib vs TNFi.
Methods: Pts with RA aged ≥50 years and with ≥1 additional CV risk factor received tofacitinib 5 mg (N=1,455) or 10 mg (N=1,456) BID, or TNFi (N=1,451). Extended MACE endpoints were based on MACE-3 and sequentially added adjudicated ischemic CV events (ie hospitalization for unstable angina [MACE-4], coronary revascularization procedures [MACE-5], transient ischemic attack [MACE-6], and peripheral vascular disease [MACE-7]), hospitalization for heart failure (HF; MACE-8), and VTE (MACE-8 plus VTE). Hazard ratios (HRs; time to first event analysis) were evaluated with tofacitinib vs TNFi for extended MACE endpoints (risk period up to first event of aggregated CV events) and for individual component endpoints (risk period up to first event of individual CV events), separately. Subgroup analyses by history of ASCVD were performed.
Results: For extended endpoints of adjudicated ischemic CV events (ie MACE-4 to -7), HRs with tofacitinib vs TNFi were similar to HRs for MACE-3 (Fig 1). Risk of MACE-8 was similar with combined tofacitinib doses vs TNFi (HR [95% confidence intervals (CI)] 1.08 [0.81, 1.44]). Risk of MACE-8 plus VTE was similar with tofacitinib 5 mg BID vs TNFi (HR 1.12 [0.82, 1.52]), but higher with tofacitinib 10 mg BID vs TNFi (HR 1.38 [1.02, 1.85]) (Fig 1). Risk of MI appeared higher with tofacitinib vs TNFi (HR 1.74 [0.89, 3.41], combined doses), but risk of other individual adjudicated CV events was generally similar (Fig 2). Across extended MACE definitions (ie up to MACE-8), risk appeared higher with tofacitinib vs TNFi in pts with history of ASCVD (Fig 3).
Conclusion: In ORAL Surveillance, risk of a composite of all ischemic CV events and HF (ie MACE-8) did not appear different with tofacitinib vs TNFi. However, across extended MACE endpoints risk was numerically higher with tofacitinib vs TNFi in pts with a history of ASCVD. The totality of CV risk (ie MACE-8 plus VTE) was higher with tofacitinib 10 mg BID vs TNFi, driven by an increase in VTE events. Limitations include low numbers of individual CV events and not considering severity/frequency of multiple events. These data highlight the need for a better understanding of risk of individual CV events in pts with RA.
Ytterberg et al. N Engl J Med 2022; 386: 316–26
Charles-Schoeman et al. Ann Rheum Dis 2022: ard-2022-222259. Epub ahead of print
Study sponsored by Pfizer. Medical writing support was provided by L Hogarth, CMC Connect, and funded by Pfizer.
.jpg)


Disclosures: M. Buch, AbbVie, Eli Lilly, Gilead Sciences, Pfizer Inc, UCB; D. Bhatt, Angio Wave, Bayer, Boehringer-Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, Myokardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences and Stasys, Bristol-Myers Squibb, High Enroll, Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Beren, Boston Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Javelin, Lexicon, Eli Lilly, Medtronic, Merck, Moderna, Myokardia, Novartis, Owkin, Pfizer Inc, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi-Regeneron, Stasys, Synaptic, The Medicines Company and 89bio, AngioWave (stock options), Boston VA Research Institute, DRS.LINQ (stock options), The Society of Cardiovascular Patient Care, TobeSoft, American Heart Association Quality Oversight Committee, Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute), The PORTICO trial, (funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, The Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), The ABILITY-DM trial (funded by Concept Medical), The Population Health Research Institute, Rutgers University (for the NIH-funded MINT trial), Deputy Editor for Clinical Cardiology, Trustee for the American College of Cardiology, American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Belvoir Publications (Editor in Chief, Harvard Heart Letter), The Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), The Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute, Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), The Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee), Elsevier (Editor, Braunwald’s Heart Disease), Named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Phillips, SpectraWAVE, Svelte, Vascular Solutions, Conducts unfunded research with FlowCo and Takeda; C. Charles-Schoeman, AbbVie, Gilead Sciences, Pfizer Inc, Sanofi-Regeneron, Bristol-Myers Squibb; J. Giles, AbbVie, Bristol-Myers Squibb, Eli Lilly, Genentech, Gilead Sciences, UCB, Pfizer Inc; T. Mikuls, Gilead Sciences, Horizon, Sanofi-Regeneron, Pfizer Inc, Bristol-Myers Squibb; G. Koch, University of North Carolina at Chapel Hill, AbbVie, Acceleron, Amgen, Arena, AstraZeneca, Cytokinetics, Eli Lilly, Gilead Sciences, GlaxoSmithKlein, Huya Bioscience International, Johnson & Johnson, Landos Biopharma, Merck, Momentum, Novartis, Otsuka, Pfizer Inc, Sanofi-Regeneron, vTv Therapeutics, IQVIA; S. Ytterberg, Corbus Pharmaceuticals, Janssen, Kezar Life Sciences, Pfizer Inc; E. Nagy, Pfizer Ltd; H. Jo, Syneos Health, Pfizer Inc; K. Kwok, Pfizer Inc; C. Connell, Pfizer Inc; K. Masri, Pfizer Inc; A. Yndestad, Pfizer Inc.

