Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1507: Diagnostic Delay of More Than 6 Months Contributes to Poor Patient-Reported Outcome Measures in Psoriatic Arthritis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GG
George Gondo, MA
National Psoriasis Foundation
Portland, OR, United States
Abstract Poster Presenter(s)
Neel Tapryal1, George Gondo2, M. Elaine Husni3, Alice Gottlieb4 and Joseph Merola5, 1Cleveland Clinic, Rocky River, OH, 2National Psoriasis Foundation, Portland, OR, 3Cleveland Clinic, Cleveland, OH, 4Department of Dermatology, The Icahn School of Medicine at Mount Sinai, New York, NY, 5Harvard Medical School, Brigham and Women's Hospital, Boston, MA
Background/Purpose: Psoriatic arthritis (PsA) is a type of inflammatory arthritis in which the time between symptom onset and a diagnosis of PsA is highly variable. In a high proportion of psoriasis patients, skin disease precedes joint symptoms by many years. Timely detection of PsA is crucial as a delay in diagnosis can lead to significant impairment in quality of life including loss of functional capacity, limitation in social activities, and depression. There is a lack of data on the magnitude of these impairments in relation to the time lag of PsA diagnosis. In this study, we aim to correlate the severity of various patient-reported outcome measures with the time period between PsA symptoms onset and diagnosis.
Methods: A cross-sectional survey of a random sample of individuals with PsA was conducted between 2019 – 2021 (N=4,019). Respondents self-reported time between PsA symptoms and diagnosis. Impact of skin disease on quality of life was assessed using the Dermatology Life Quality Index (DLQI). Patient Reported Outcome Measurement and Information System (PROMIS), and Ability to Participate in Social Roles and Activities (SF-4a) assessed ability to participate in social roles and activities. Depression was assessed with the Patient Health Questionnaire -2 (PHQ-2). Impact of PsA on quality of life and PsA symptoms severity were assessed with the Psoriatic Arthritis Impact of Disease (PsAID-9). Chi-square tests of independence were used to assess whether severity of patient outcomes were associated with time between PsA symptom onset and diagnosis. Analysis of Covariance assessed differences in outcomes based on time between PsA symptom onset and diagnosis while controlling for age and body mass index.
Results: 2,196 patients (mean age 57 years, 85.3% white) with PsA completed the survey. Chi-square results suggest that increased time between PsA symptom onset and diagnosis of PsA were associated with a greater incidence of depression, limitation in ability to participate in social roles and activities, and greater impact of PsA on quality of life (Table 1). Likelihood of depression progressively increased when time between PsA symptom onset and diagnosis was beyond 6 months 28.6% at < 6 months vs 29.6% at 7-12 months vs 38.1% at 13-24 months vs 35.4% at >2 years (p < 0.01). Individuals with more than 5 months between PsA symptoms onset and diagnosis reported higher rates of experiencing moderate limitation in their ability to participate in social roles and activities (22.9% at < 6 months; 29.2% at 7-12 months; 34.0% at 13-24 months; 35.3% at >2 years; p < 0.001). Unacceptable PsA symptom rates (PsAID score >4) increased with time between PsA symptom onset and diagnosis (74.7% at < 6 months vs 76.4% at 7-12 months vs 80.8% at 13-24 months vs 81.6% at >2 years; p < 0.05). When controlling for age and BMI, individuals with 6 months or less between PsA symptom onset and diagnosis reported better outcomes than those with > 6 months (Table 2).
Conclusion: Our study demonstrated that even a 6-month delay in diagnosis of PsA from symptom onset contributes to worse patient-reported outcomes measures on overall quality of life. These results underscore the importance of early diagnosis of PsA as it is essential in optimizing the patient’s quality of life.
.jpg) Associate between time between PsA symptom onset and diagnosis compared and patient outcomes
Associate between time between PsA symptom onset and diagnosis compared and patient outcomes
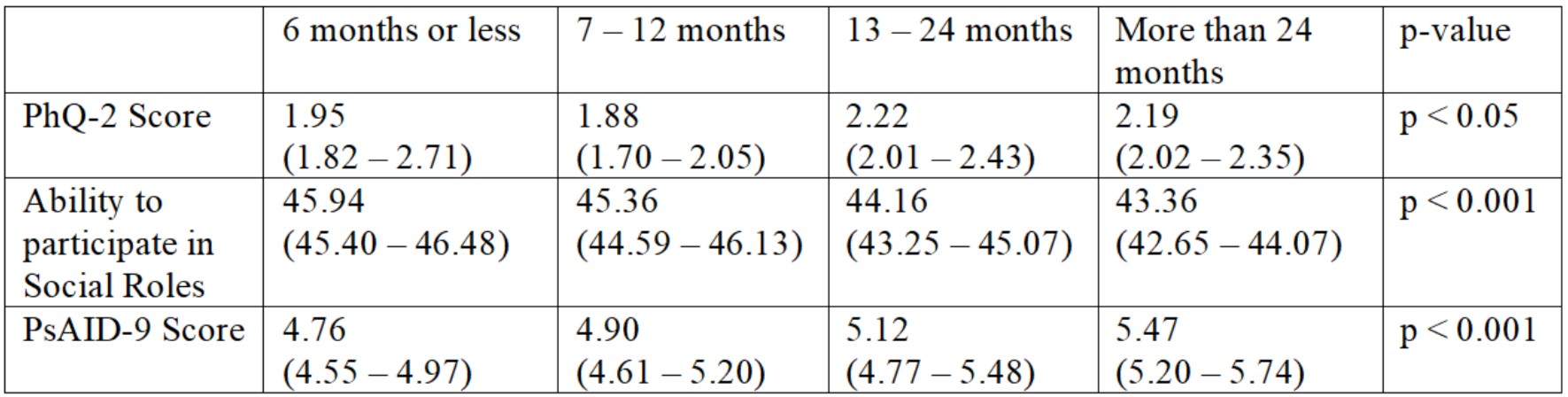 Comparing outcomes based on time between PsA symptom onset and diagnoses while controlling for age and BMI
Comparing outcomes based on time between PsA symptom onset and diagnoses while controlling for age and BMI
Disclosures: N. Tapryal, None; G. Gondo, None; M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; A. Gottlieb, Amgen, AnaptsysBio, Avortres Therapeutics, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Sun Pharmaceuticals Industries, UCB Pharma, Xbiotech, Ortho; J. Merola, AbbVie, Biogen, BMS, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, UCB Pharma, Arena, Avotres, EMD, LEO Pharma, Merck, Regeneron, Sanofi.
Background/Purpose: Psoriatic arthritis (PsA) is a type of inflammatory arthritis in which the time between symptom onset and a diagnosis of PsA is highly variable. In a high proportion of psoriasis patients, skin disease precedes joint symptoms by many years. Timely detection of PsA is crucial as a delay in diagnosis can lead to significant impairment in quality of life including loss of functional capacity, limitation in social activities, and depression. There is a lack of data on the magnitude of these impairments in relation to the time lag of PsA diagnosis. In this study, we aim to correlate the severity of various patient-reported outcome measures with the time period between PsA symptoms onset and diagnosis.
Methods: A cross-sectional survey of a random sample of individuals with PsA was conducted between 2019 – 2021 (N=4,019). Respondents self-reported time between PsA symptoms and diagnosis. Impact of skin disease on quality of life was assessed using the Dermatology Life Quality Index (DLQI). Patient Reported Outcome Measurement and Information System (PROMIS), and Ability to Participate in Social Roles and Activities (SF-4a) assessed ability to participate in social roles and activities. Depression was assessed with the Patient Health Questionnaire -2 (PHQ-2). Impact of PsA on quality of life and PsA symptoms severity were assessed with the Psoriatic Arthritis Impact of Disease (PsAID-9). Chi-square tests of independence were used to assess whether severity of patient outcomes were associated with time between PsA symptom onset and diagnosis. Analysis of Covariance assessed differences in outcomes based on time between PsA symptom onset and diagnosis while controlling for age and body mass index.
Results: 2,196 patients (mean age 57 years, 85.3% white) with PsA completed the survey. Chi-square results suggest that increased time between PsA symptom onset and diagnosis of PsA were associated with a greater incidence of depression, limitation in ability to participate in social roles and activities, and greater impact of PsA on quality of life (Table 1). Likelihood of depression progressively increased when time between PsA symptom onset and diagnosis was beyond 6 months 28.6% at < 6 months vs 29.6% at 7-12 months vs 38.1% at 13-24 months vs 35.4% at >2 years (p < 0.01). Individuals with more than 5 months between PsA symptoms onset and diagnosis reported higher rates of experiencing moderate limitation in their ability to participate in social roles and activities (22.9% at < 6 months; 29.2% at 7-12 months; 34.0% at 13-24 months; 35.3% at >2 years; p < 0.001). Unacceptable PsA symptom rates (PsAID score >4) increased with time between PsA symptom onset and diagnosis (74.7% at < 6 months vs 76.4% at 7-12 months vs 80.8% at 13-24 months vs 81.6% at >2 years; p < 0.05). When controlling for age and BMI, individuals with 6 months or less between PsA symptom onset and diagnosis reported better outcomes than those with > 6 months (Table 2).
Conclusion: Our study demonstrated that even a 6-month delay in diagnosis of PsA from symptom onset contributes to worse patient-reported outcomes measures on overall quality of life. These results underscore the importance of early diagnosis of PsA as it is essential in optimizing the patient’s quality of life.
.jpg) Associate between time between PsA symptom onset and diagnosis compared and patient outcomes
Associate between time between PsA symptom onset and diagnosis compared and patient outcomes Comparing outcomes based on time between PsA symptom onset and diagnoses while controlling for age and BMI
Comparing outcomes based on time between PsA symptom onset and diagnoses while controlling for age and BMIDisclosures: N. Tapryal, None; G. Gondo, None; M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; A. Gottlieb, Amgen, AnaptsysBio, Avortres Therapeutics, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Sun Pharmaceuticals Industries, UCB Pharma, Xbiotech, Ortho; J. Merola, AbbVie, Biogen, BMS, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, UCB Pharma, Arena, Avotres, EMD, LEO Pharma, Merck, Regeneron, Sanofi.

