Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1332–1359) Patient Outcomes, Preferences, and Attitudes Poster II
1354: Early Real-World Effectiveness of Upadacitinib in Rheumatoid Arthritis Using Patient-Reported Outcomes Collected via Mobile Application
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LH
Leslie Harrold, MD, MPH
CorEvitas
Northborough, MA, United States
Abstract Poster Presenter(s)
Leslie Harrold1, Patrick Zueger2, William Benjamin Nowell3, Taylor Blachley1, Paul Lakin1, David Curtis4, Laura Stradford3, Shilpa Venkatachalam5, Namita Tundia2 and Pankaj Patel2, 1CorEvitas, LLC, Waltham, MA, 2AbbVie, Inc., North Chicago, IL, 3Global Healthy Living Foundation, Nyack, NY, 4Global Healthy Living Foundation, Upper Nyack, NY, 5Global Healthy Living Foundation, New York, NY
Background/Purpose: Little is known about the impact of upadacitinib (UPA) in rheumatoid arthritis (RA) during the initial weeks of therapy in real-world clinical practice. Our objective was to evaluate the impact of UPA on patient-reported outcomes (PROs) in patients with RA during the first 12 weeks of treatment using a mobile health application (app).
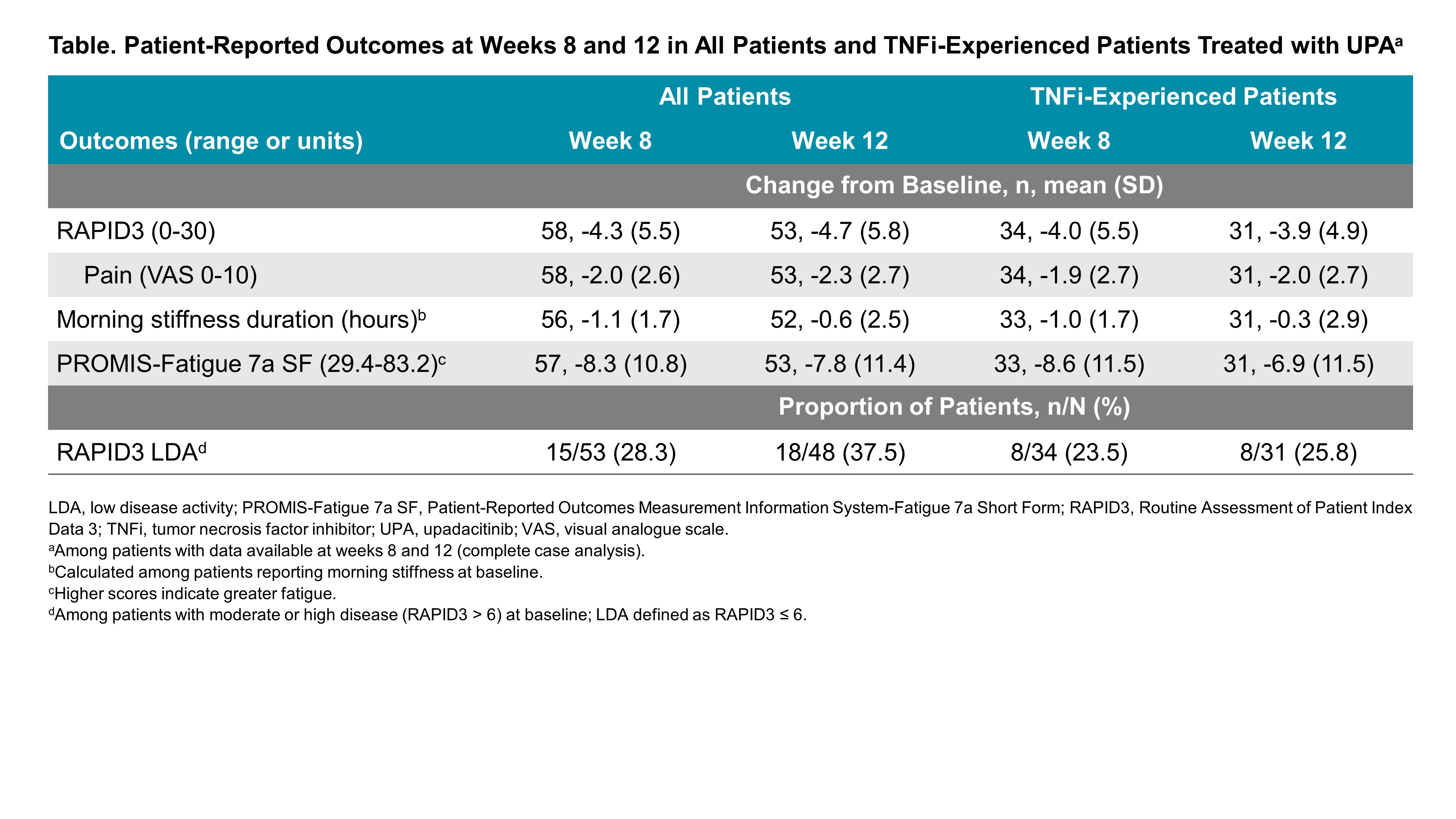
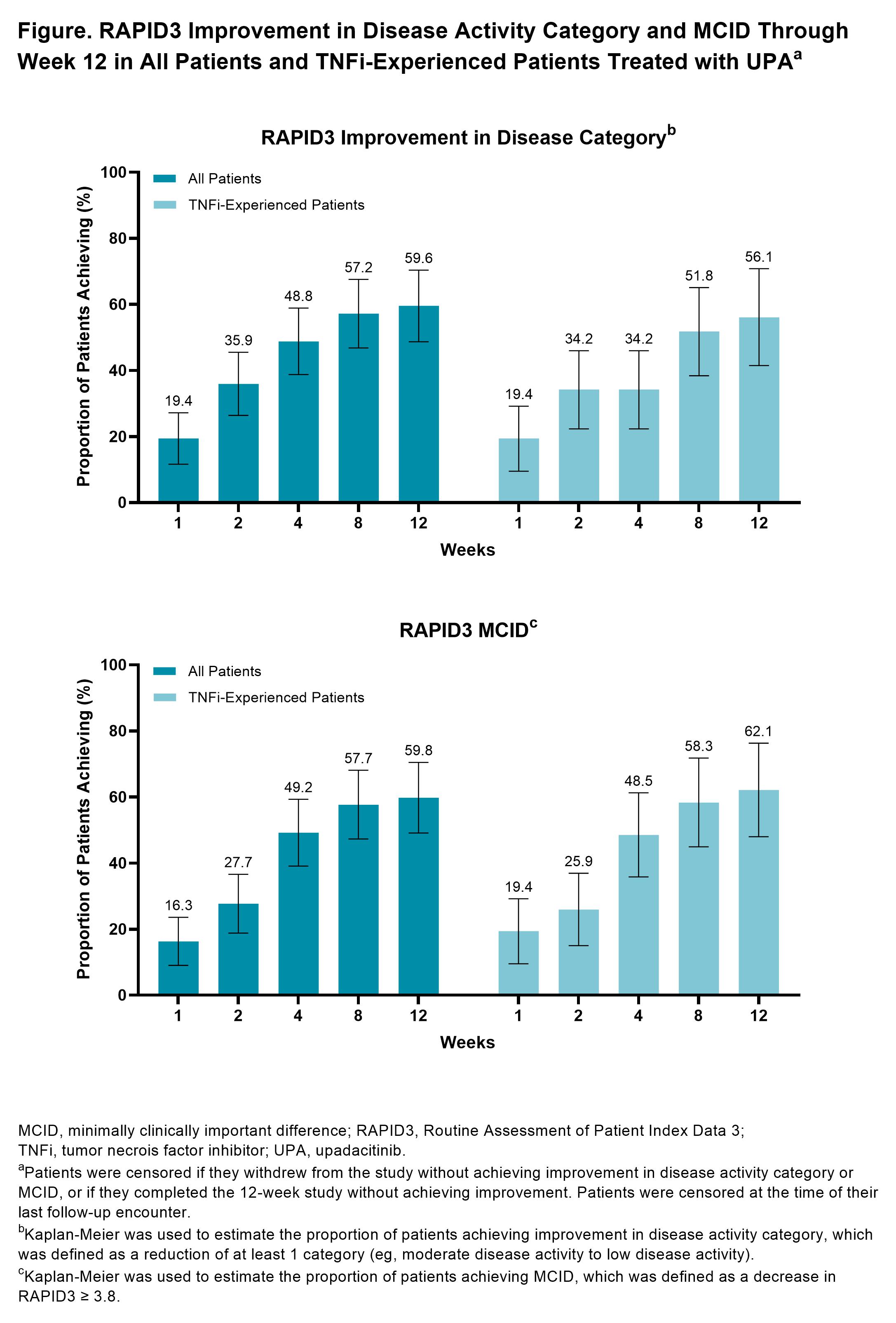
Methods: Participating rheumatologists from the CorEvitas RA Registry recruited patients across 17 sites between July 2020 and October 2021 who were initiating UPA treatment. A modified version of the ArthritisPower app was used to collect PROs, including RAPID3, PROMIS Fatigue-7a Short Form, and duration of morning joint stiffness, at baseline and weeks 1-4, 8, and 12. PROs are summarized as mean change from baseline (SD) for continuous outcomes (RAPID3 [including pain], PROMIS Fatigue, duration of morning stiffness) and as proportion of patients achieving response (RAPID3 low disease activity [LDA; defined as RAPID3 ≤ 6]) for binary outcomes. The treatment satisfaction questionnaire for medication (TSQM-9; range: 0-100 for effectiveness, convenience, and overall satisfaction subscales) and compliance questionnaire for rheumatology (CQR; range: 0-20) were assessed at weeks 8 and 12 and are summarized as mean (SD). RAPID3 responses over time were assessed using Kaplan-Meier estimation to determine the proportion of patients achieving disease activity improvement (defined as a reduction of at least one RAPID3 disease activity category) and minimally clinically important difference (MCID; defined as a decrease ≥ 3.8). Results were analyzed for all patients initiating UPA treatment, as well as for a subsample of TNF inhibitor (TNFi)-experienced patients with moderate to severe disease at baseline (defined as RAPID3 > 6).
Results: In total, 103 RA patients initiating UPA (62.1% TNFi-experienced) were included in this analysis. At initiation, the mean age of patients was 59.9 years old, most were female (81.6%), and mean (SD) RAPID3 was 14.9 (5.2). At week 12, 53 patients (51.4%) completed the study and provided PRO data via the app. Amongst all patients initiating UPA treatment, improvements in RAPID3, pain, fatigue, and morning stiffness were observed at weeks 8 and 12 (Table). At week 12, 37.5% of patients achieved RAPID3 LDA. Starting at week 1, improvements in RAPID3 disease activity and achievement of MCID were reported, with nearly 50% of patients achieving these outcomes by week 4 and 60% by week 12 (Figure). Similar or slightly attenuated results were observed in patients with prior TNFi experience. Amongst all patients initiating UPA treatment, mean (SD) responses at week 12 for TSQM-9 for the effectiveness subscale was 66.8 (18.7), for convenience 83.6 (13.8), and for overall satisfaction 68.5 (20.3). Mean (SD) CQR at week 12 for all patients was 17.5 (2.1).
Conclusion: In this real-world cohort of RA patients, treatment with UPA was associated with improvement in RAPID3, pain, fatigue, and morning stiffness regardless of prior TNFi experience. Improvement in RAPID3 patient-reported disease activity was observed as early as week 1, with continued improvement reported through week 12.
Disclosures: L. Harrold, Bristol-Myers Squibb(BMS), CorEvitas, LLC, AbbVie Inc., Roche; P. Zueger, AbbVie; W. Nowell, Global Healthy Living Foundation, AbbVie Inc., Amgen, Eli Lilly; T. Blachley, CorEvitas; P. Lakin, CorEvitas, LLC; D. Curtis, Global Healthy Living Foundation; L. Stradford, Global Healthy Living Foundation; S. Venkatachalam, None; N. Tundia, AbbVie Inc.; P. Patel, AbbVie, Inc..
Background/Purpose: Little is known about the impact of upadacitinib (UPA) in rheumatoid arthritis (RA) during the initial weeks of therapy in real-world clinical practice. Our objective was to evaluate the impact of UPA on patient-reported outcomes (PROs) in patients with RA during the first 12 weeks of treatment using a mobile health application (app).
Methods: Participating rheumatologists from the CorEvitas RA Registry recruited patients across 17 sites between July 2020 and October 2021 who were initiating UPA treatment. A modified version of the ArthritisPower app was used to collect PROs, including RAPID3, PROMIS Fatigue-7a Short Form, and duration of morning joint stiffness, at baseline and weeks 1-4, 8, and 12. PROs are summarized as mean change from baseline (SD) for continuous outcomes (RAPID3 [including pain], PROMIS Fatigue, duration of morning stiffness) and as proportion of patients achieving response (RAPID3 low disease activity [LDA; defined as RAPID3 ≤ 6]) for binary outcomes. The treatment satisfaction questionnaire for medication (TSQM-9; range: 0-100 for effectiveness, convenience, and overall satisfaction subscales) and compliance questionnaire for rheumatology (CQR; range: 0-20) were assessed at weeks 8 and 12 and are summarized as mean (SD). RAPID3 responses over time were assessed using Kaplan-Meier estimation to determine the proportion of patients achieving disease activity improvement (defined as a reduction of at least one RAPID3 disease activity category) and minimally clinically important difference (MCID; defined as a decrease ≥ 3.8). Results were analyzed for all patients initiating UPA treatment, as well as for a subsample of TNF inhibitor (TNFi)-experienced patients with moderate to severe disease at baseline (defined as RAPID3 > 6).
Results: In total, 103 RA patients initiating UPA (62.1% TNFi-experienced) were included in this analysis. At initiation, the mean age of patients was 59.9 years old, most were female (81.6%), and mean (SD) RAPID3 was 14.9 (5.2). At week 12, 53 patients (51.4%) completed the study and provided PRO data via the app. Amongst all patients initiating UPA treatment, improvements in RAPID3, pain, fatigue, and morning stiffness were observed at weeks 8 and 12 (Table). At week 12, 37.5% of patients achieved RAPID3 LDA. Starting at week 1, improvements in RAPID3 disease activity and achievement of MCID were reported, with nearly 50% of patients achieving these outcomes by week 4 and 60% by week 12 (Figure). Similar or slightly attenuated results were observed in patients with prior TNFi experience. Amongst all patients initiating UPA treatment, mean (SD) responses at week 12 for TSQM-9 for the effectiveness subscale was 66.8 (18.7), for convenience 83.6 (13.8), and for overall satisfaction 68.5 (20.3). Mean (SD) CQR at week 12 for all patients was 17.5 (2.1).
Conclusion: In this real-world cohort of RA patients, treatment with UPA was associated with improvement in RAPID3, pain, fatigue, and morning stiffness regardless of prior TNFi experience. Improvement in RAPID3 patient-reported disease activity was observed as early as week 1, with continued improvement reported through week 12.


Disclosures: L. Harrold, Bristol-Myers Squibb(BMS), CorEvitas, LLC, AbbVie Inc., Roche; P. Zueger, AbbVie; W. Nowell, Global Healthy Living Foundation, AbbVie Inc., Amgen, Eli Lilly; T. Blachley, CorEvitas; P. Lakin, CorEvitas, LLC; D. Curtis, Global Healthy Living Foundation; L. Stradford, Global Healthy Living Foundation; S. Venkatachalam, None; N. Tundia, AbbVie Inc.; P. Patel, AbbVie, Inc..

