Back
Poster Session C
Vasculitis
Session: (1543–1578) Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
1547: Effectiveness of Interleukin-6 Receptor Inhibitors for Polymyalgia Rheumatica
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Jeffrey Curtis, MD, MS, MPH
University of Alabama at Birmingham
Hoover, AL, United States
Abstract Poster Presenter(s)
Jeffrey Curtis1, Kerri Ford2, Stefano Fiore3, Danielle Isaman4, Lita Araujo5, Natalia Petruski-Ivleva6 and Fenglong Xie7, 1Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, Birmingham, AL, 2Sanofi, Destin, FL, 3Sanofi, Bridgewater, NJ, 4Sanofi, Chapel Hill, NC, 5Sanofi, Cambridge, MA, 6Sanofi, Unknown, 7University of Alabama at Birmingham, Birmingham, AL
Background/Purpose: Interleukin-6 receptor (IL-6R) inhibition has been shown to be effective in giant cell arteritis but data are limited in polymyalgia rheumatica (PMR). We conducted a retrospective study to evaluate effectiveness of IL-6R inhibitors (IL-6Ri; sarilumab or tocilizumab) in 3rd-line and 2nd-line treatment of glucocorticoid (GC)-refractory PMR patients compared to conventional immunomodulatory (cIM) therapy.
Methods: Adults with PMR, defined as 1 inpatient or 2 outpatient claims with a PMR diagnosis ≥30 days apart, were identified from national fee-for-service Medicare (100% sample) data between 3/29/2016- to 6/30/2020. Included were those who initiated IL-6Ri or cIM as 2nd-line and 3rd-line therapy for PMR and had continuous enrollment for 180 days prior to therapy initiation (baseline). For 3rd-line cohort, the index date was date of switch from cIM to IL-6Ri for the IL-6Ri group and switch from one cIM to another cIM for the cIM group. For 2nd-line cohort, the index date was the date of initiation of IL-6Ri with no prior cIM for the IL-6Ri group and new use of cIM for the cIM group. A 180-day washout period for the index drug was applied to all cohorts. Patients were on GC on index date (≤25mg/day) and excluded if they had evidence of seropositive rheumatoid arthritis, other inflammatory arthritis or connective tissue disease, multiple sclerosis, or malignancy. Patients on IL-6Ri and cIM were matched 1:1 on age, sex, and GC dose category, and propensity score matching was used to adjust for potential confounders.
Outcomes assessed using adjusted Cox proportional hazards models included GC discontinuation; GC discontinuation or low dose GC (< 2mg/day); and non-persistence (discontinuation of IL-6Ri or cIM, or switch) in the 2nd-line cohort, adjusting for residually imbalanced factors after matching. Censoring occurred at 1 year, death, 60 days before end of enrollment, or addition of comparator PMR therapy with additional censoring applied in sensitivity analysis for drug discontinuation and/or switching.
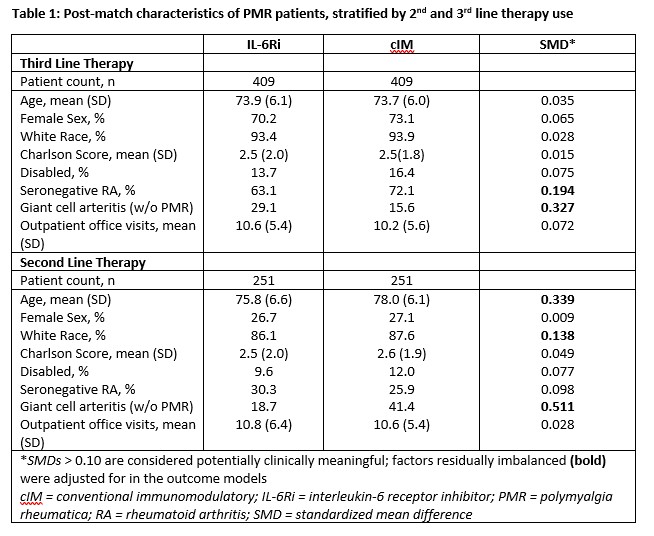
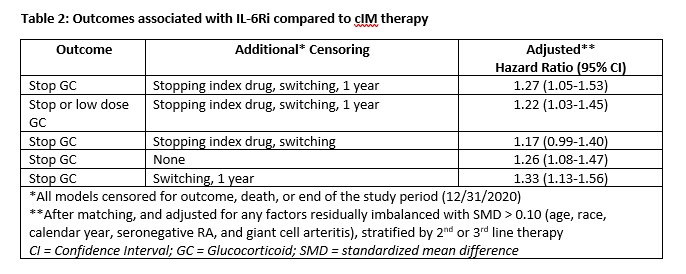
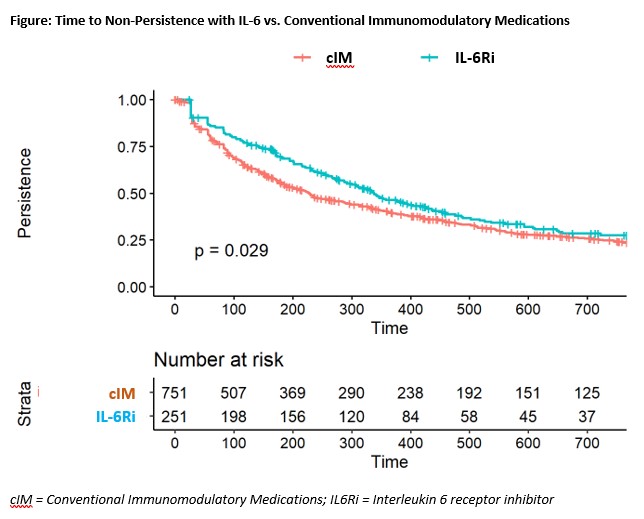
Results: After matching, 409 3rd-line and 251 2nd-line patients were included in the analysis. Post-match, patient characteristics were generally well balanced between cohorts, with small differences between exposure groups (Table 1). Median time from first PMR diagnosis to start of follow-up was approximately 1.75 years (3rd-line) and 0.90 years (2nd-line) and similar between groups. After matching and adjustment, patients receiving IL-6Ri were more likely to achieve GC discontinuation or low dose GC, compared to cIM patients (Table 2). Time to discontinuation (Figure) was significantly greater for IL-6Ri vs. cIM patients (p=0.029).
Conclusion: IL-6Ri therapy was more effective as a steroid sparing agent compared to cIM therapy for adult patients with PMR.
Disclosures: J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; K. Ford, Sanofi; S. Fiore, Sanofi; D. Isaman, Sanofi; L. Araujo, Sanofi; N. Petruski-Ivleva, Sanofi; F. Xie, Bendcare.
Background/Purpose: Interleukin-6 receptor (IL-6R) inhibition has been shown to be effective in giant cell arteritis but data are limited in polymyalgia rheumatica (PMR). We conducted a retrospective study to evaluate effectiveness of IL-6R inhibitors (IL-6Ri; sarilumab or tocilizumab) in 3rd-line and 2nd-line treatment of glucocorticoid (GC)-refractory PMR patients compared to conventional immunomodulatory (cIM) therapy.
Methods: Adults with PMR, defined as 1 inpatient or 2 outpatient claims with a PMR diagnosis ≥30 days apart, were identified from national fee-for-service Medicare (100% sample) data between 3/29/2016- to 6/30/2020. Included were those who initiated IL-6Ri or cIM as 2nd-line and 3rd-line therapy for PMR and had continuous enrollment for 180 days prior to therapy initiation (baseline). For 3rd-line cohort, the index date was date of switch from cIM to IL-6Ri for the IL-6Ri group and switch from one cIM to another cIM for the cIM group. For 2nd-line cohort, the index date was the date of initiation of IL-6Ri with no prior cIM for the IL-6Ri group and new use of cIM for the cIM group. A 180-day washout period for the index drug was applied to all cohorts. Patients were on GC on index date (≤25mg/day) and excluded if they had evidence of seropositive rheumatoid arthritis, other inflammatory arthritis or connective tissue disease, multiple sclerosis, or malignancy. Patients on IL-6Ri and cIM were matched 1:1 on age, sex, and GC dose category, and propensity score matching was used to adjust for potential confounders.
Outcomes assessed using adjusted Cox proportional hazards models included GC discontinuation; GC discontinuation or low dose GC (< 2mg/day); and non-persistence (discontinuation of IL-6Ri or cIM, or switch) in the 2nd-line cohort, adjusting for residually imbalanced factors after matching. Censoring occurred at 1 year, death, 60 days before end of enrollment, or addition of comparator PMR therapy with additional censoring applied in sensitivity analysis for drug discontinuation and/or switching.
Results: After matching, 409 3rd-line and 251 2nd-line patients were included in the analysis. Post-match, patient characteristics were generally well balanced between cohorts, with small differences between exposure groups (Table 1). Median time from first PMR diagnosis to start of follow-up was approximately 1.75 years (3rd-line) and 0.90 years (2nd-line) and similar between groups. After matching and adjustment, patients receiving IL-6Ri were more likely to achieve GC discontinuation or low dose GC, compared to cIM patients (Table 2). Time to discontinuation (Figure) was significantly greater for IL-6Ri vs. cIM patients (p=0.029).
Conclusion: IL-6Ri therapy was more effective as a steroid sparing agent compared to cIM therapy for adult patients with PMR.



Disclosures: J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; K. Ford, Sanofi; S. Fiore, Sanofi; D. Isaman, Sanofi; L. Araujo, Sanofi; N. Petruski-Ivleva, Sanofi; F. Xie, Bendcare.

