Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1542: Exploratory Clinical Subgroup Clustering in Systemic Sclerosis - Results from the Indian Progressive Systemic Sclerosis Registry
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SP
Shery Susan Philip, MD, MBBS
St. John’s Medical College Hospital
Bangalore, Karnataka, India
Abstract Poster Presenter(s)
Shery Susan Philip1, Ramya Janardana1, Revanesh S Mirji1, Chengappa Kavadichanda2, Devender Bairwa3, Geetabali Sircar4, Parasar Ghosh5, Anupam Wakhlu6, Padmanabha Shenoy7, Sumithra Selvam8 and Vineeta Shobha1, 1St. John’s Medical College Hospital, Bangalore, India, 2JIPMER, Pondicherry, Puducherry, India, 3Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, Puducherry, India, 4Institute of Postgraduate Medical Education and Research, Kolkata, West Bengal, India, 5Govt of West Bengal, Kolkata, India, Kolkata, West Bengal, India, 6Apollo Medics Super Speciality Hospitals, Lucknow, India, 7Centre for Arthritis and Rheumatism Excellence (CARE), Cochi, India, 8Division of Epidemiology and Biostatistics, St. John’s Research Institute, St. John's Medical College Hospital, Bangalore, India
Background/Purpose: Classification of systemic sclerosis (SSc) into subsets has been a challenge due to it's heterogeneity. This study attempts to identify SSc subsets based on antibody and clinical characteristics in the Indian population that may help stratify the risk of distinct clinical presentations.
Methods: We included 430 SSc patients from the multicentric Indian Progressive Systemic Sclerosis Registry (IPSSR) enrolled between 2019 and 2021. Patients who did not satisfy the ACR-EULAR SSc classification criteria or did not have antibody reports were excluded. Two-step cluster analysis was performed to identify subgroups based on immunological and clinical characteristics.
Results: We differentiated 4 clusters based on autoantibody and clinical features. Baseline characteristics are described in table 1. Distribution of clinical characteristics and autoantibodies across the clusters is described in figure 1. Cluster 1 had high prevalence of anti-centromere antibody (ACA) and clusters 2, 3 and 4 had anti-topoisomerase antibody (topo I). Among the topo I positive clusters, cluster 2 had limited skin pattern, while clusters 3 and 4 had diffuse skin disease, with MRSS scores significantly differing between them. Cluster 4 had younger age at disease onset (p< 0.0001), diffuse skin pattern along with large joint contractures and tendon friction rubs, and highest prevalence of musculoskeletal features (arthritis, proximal muscle weakness), GI features (diarrhea, constipation, anal incontinence, dysphagia, heart burn) and vasculopathic features (digital ulcers, pitting scars). Cluster 4 had the worst modified Rodnan skin score (MRSS) (p< 0.0001), patient (p< 0.0001) and physician global assessment (p< 0.0001) (PtGA, PGA respectively), indicating that this group represented a severe disease subset. BMI was also significantly lower in cluster 4, suggesting a consequence of severe disease (p=0.018). However, other clinical manifestations seem to be similar among clusters 2 and 3, and PGA and PtGA were not significantly different between them. Cluster 1 represented a mild subset with older age at onset (p< 0.0001), limited skin pattern, and the least MRSS (p< 0.0001), denoting least severe skin disease. The prevalence of interstitial lung disease (ILD) was similar in all 3 clusters with topo I positivity (p< 0.0001), indicating strong association of topo I with ILD irrespective of the extent of skin involvement (Figure 2).
Conclusion: We identified 3 subsets within the topo I positive group, reflecting a spectrum of cutaneous severity and organ involvement that may help in risk stratification to predict outcomes/ prognosis. Unlike previous consideration, these results suggest that topo I positivity is not synonymous with only severe or diffuse cutaneous disease. Cluster 4 was found to have the most severe disease in terms of MRSS, global assessments and clinical features. Interestingly, topo I was strongly associated with ILD irrespective of the extent of skin involvement. However, these findings have to be validated further in larger, individual cohorts.
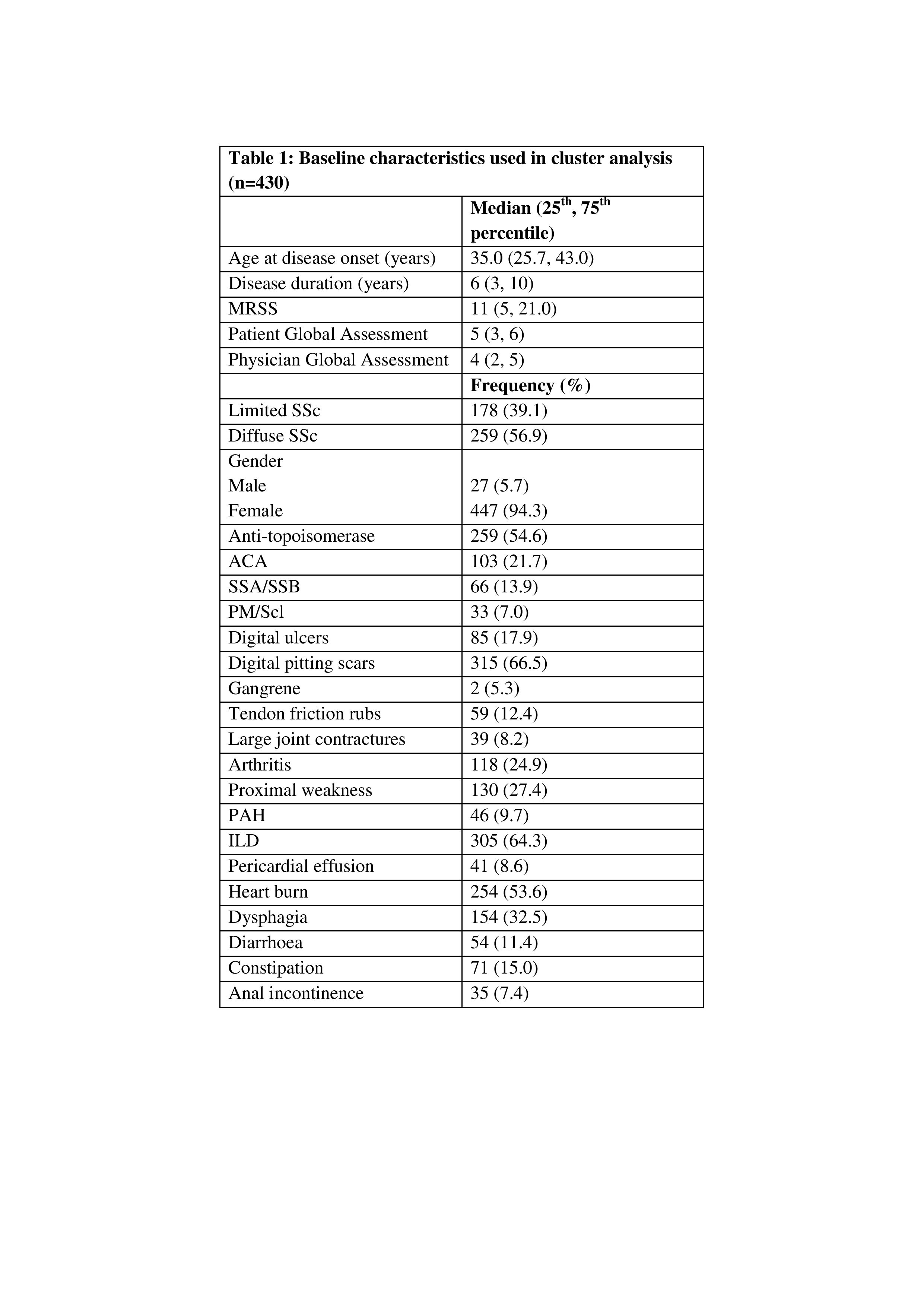 Table 1: Baseline characteristics used in cluster analysis (n=430).
Table 1: Baseline characteristics used in cluster analysis (n=430).
Abbreviations: MRSS-Modified Rodnan Skin Score, SSc-Systemic Sclerosis, ACA-Anti-centromere antibody, SSA/SSB-Anti-Sjogren's Syndrome (Ro) A/B(La), PM/Scl-Polymyositis/Scleroderma, PAH-Pulmonary Arterial Hypertension, ILD-Interstitial Lung Disease.
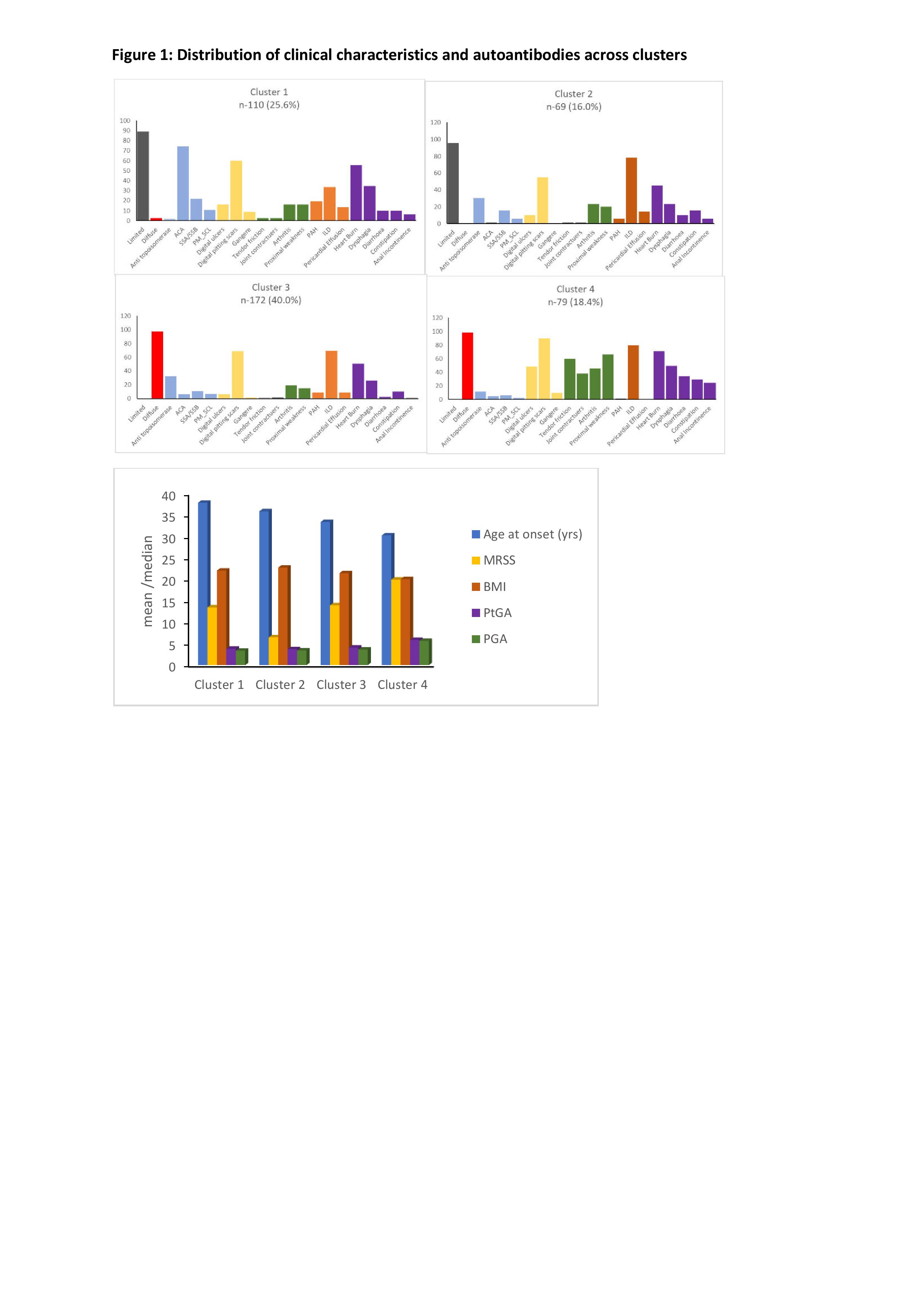 Figure 1: Distribution of clinical characteristics and autoantibodies across clusters.
Figure 1: Distribution of clinical characteristics and autoantibodies across clusters.
Abbreviations: ACA-Anti-centromere antibody, SSA/SSB-Anti-Sjogren's Syndrome (Ro) A/B(La), PAH-Pulmonary Arterial Hypertension, ILD-Interstitial Lung Disease, PM_SCL-Polymyositis/Scleroderma, MRSS-Modified Rodnan Skin Score, BMI-Body Mass Index, PtGA-Patient Global Assessment, PGA-Physician Global Assessment.
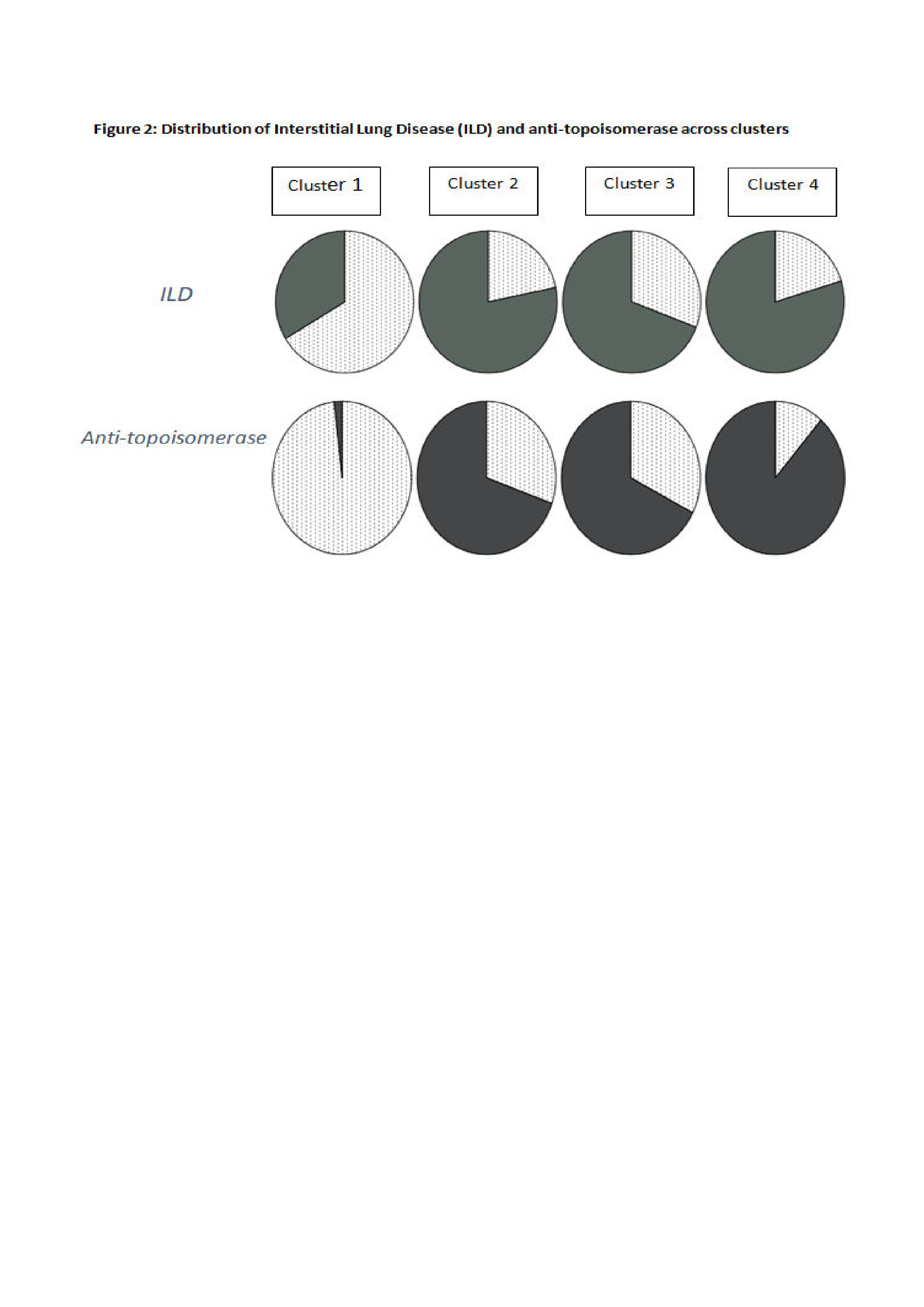 Figure 2: Distribution of interstitial lung disease (ILD) and anti-topoisomerase across clusters.
Figure 2: Distribution of interstitial lung disease (ILD) and anti-topoisomerase across clusters.
Disclosures: S. Philip, None; R. Janardana, None; R. Mirji, None; C. Kavadichanda, None; D. Bairwa, None; G. Sircar, None; P. Ghosh, None; A. Wakhlu, None; P. Shenoy, None; S. Selvam, None; V. Shobha, None.
Background/Purpose: Classification of systemic sclerosis (SSc) into subsets has been a challenge due to it's heterogeneity. This study attempts to identify SSc subsets based on antibody and clinical characteristics in the Indian population that may help stratify the risk of distinct clinical presentations.
Methods: We included 430 SSc patients from the multicentric Indian Progressive Systemic Sclerosis Registry (IPSSR) enrolled between 2019 and 2021. Patients who did not satisfy the ACR-EULAR SSc classification criteria or did not have antibody reports were excluded. Two-step cluster analysis was performed to identify subgroups based on immunological and clinical characteristics.
Results: We differentiated 4 clusters based on autoantibody and clinical features. Baseline characteristics are described in table 1. Distribution of clinical characteristics and autoantibodies across the clusters is described in figure 1. Cluster 1 had high prevalence of anti-centromere antibody (ACA) and clusters 2, 3 and 4 had anti-topoisomerase antibody (topo I). Among the topo I positive clusters, cluster 2 had limited skin pattern, while clusters 3 and 4 had diffuse skin disease, with MRSS scores significantly differing between them. Cluster 4 had younger age at disease onset (p< 0.0001), diffuse skin pattern along with large joint contractures and tendon friction rubs, and highest prevalence of musculoskeletal features (arthritis, proximal muscle weakness), GI features (diarrhea, constipation, anal incontinence, dysphagia, heart burn) and vasculopathic features (digital ulcers, pitting scars). Cluster 4 had the worst modified Rodnan skin score (MRSS) (p< 0.0001), patient (p< 0.0001) and physician global assessment (p< 0.0001) (PtGA, PGA respectively), indicating that this group represented a severe disease subset. BMI was also significantly lower in cluster 4, suggesting a consequence of severe disease (p=0.018). However, other clinical manifestations seem to be similar among clusters 2 and 3, and PGA and PtGA were not significantly different between them. Cluster 1 represented a mild subset with older age at onset (p< 0.0001), limited skin pattern, and the least MRSS (p< 0.0001), denoting least severe skin disease. The prevalence of interstitial lung disease (ILD) was similar in all 3 clusters with topo I positivity (p< 0.0001), indicating strong association of topo I with ILD irrespective of the extent of skin involvement (Figure 2).
Conclusion: We identified 3 subsets within the topo I positive group, reflecting a spectrum of cutaneous severity and organ involvement that may help in risk stratification to predict outcomes/ prognosis. Unlike previous consideration, these results suggest that topo I positivity is not synonymous with only severe or diffuse cutaneous disease. Cluster 4 was found to have the most severe disease in terms of MRSS, global assessments and clinical features. Interestingly, topo I was strongly associated with ILD irrespective of the extent of skin involvement. However, these findings have to be validated further in larger, individual cohorts.
 Table 1: Baseline characteristics used in cluster analysis (n=430).
Table 1: Baseline characteristics used in cluster analysis (n=430).Abbreviations: MRSS-Modified Rodnan Skin Score, SSc-Systemic Sclerosis, ACA-Anti-centromere antibody, SSA/SSB-Anti-Sjogren's Syndrome (Ro) A/B(La), PM/Scl-Polymyositis/Scleroderma, PAH-Pulmonary Arterial Hypertension, ILD-Interstitial Lung Disease.
 Figure 1: Distribution of clinical characteristics and autoantibodies across clusters.
Figure 1: Distribution of clinical characteristics and autoantibodies across clusters.Abbreviations: ACA-Anti-centromere antibody, SSA/SSB-Anti-Sjogren's Syndrome (Ro) A/B(La), PAH-Pulmonary Arterial Hypertension, ILD-Interstitial Lung Disease, PM_SCL-Polymyositis/Scleroderma, MRSS-Modified Rodnan Skin Score, BMI-Body Mass Index, PtGA-Patient Global Assessment, PGA-Physician Global Assessment.
 Figure 2: Distribution of interstitial lung disease (ILD) and anti-topoisomerase across clusters.
Figure 2: Distribution of interstitial lung disease (ILD) and anti-topoisomerase across clusters.Disclosures: S. Philip, None; R. Janardana, None; R. Mirji, None; C. Kavadichanda, None; D. Bairwa, None; G. Sircar, None; P. Ghosh, None; A. Wakhlu, None; P. Shenoy, None; S. Selvam, None; V. Shobha, None.

