Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1497: How Does Time to Diagnosis and Gender Affect Treatment Outcomes in Patients with Ankylosing Spondylitis or Psoriatic Arthritis? - Real World Data from a German Observational Study with Secukinumab
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- UK
Uta Kiltz, PhD
Rheumazentrum Ruhrgebiet
D-44649 Herne, Germany
Abstract Poster Presenter(s)
Karolina Benesova1, Oliver Hansen2, Uta Kiltz3, Jan Brandt-Juergens4, Peter Kästner5, Elke Riechers6, Daniel Peterlik7, Christina Budden7, Annika Boas7, Stefanie Welle7 and Hans-Peter Tony8, 1University Hospital Heidelberg, Heidelberg, Germany, 2Medizinische Abteilung V - Rheumatologie, Universitätsklinikum, Heidelberg, Germany, 3Rheumazentrum Ruhrgebiet, Herne, Germany, 4Rheumatologische Schwerpunktpraxis, Berlin, Germany, 5Ambulantes Rheumazentrum, Medizinisches Versorgungszentrum, Erfurt, Germany, 6Medizinische Hochschule Hannover, Hannover, Germany, 7Novartis Pharma GmbH, Nürnberg, Germany, 8Medizinische Klinik II - Rheumatologie/Immunologie, Universitätsklinikum Würzburg, Würzburg, Germany
Background/Purpose: In both, ankylosing spondylitis (AS) and psoriatic arthritis (PsA), women typically have a longer delay in diagnosis.1,2 There is scientific evidence that prognosis for AS and PsA improves when diagnosed early. The German non-interventional study AQUILA provides real-world data on the influence of time to diagnosis and gender on treatment outcomes under secukinumab, a fully human monoclonal antibody that selectively inhibits interleukin-17A.The aims of this interim analysis are to describe selected baseline (BL) demographics of AS and PsA patients (pts) and to evaluate the impact of time to diagnosis and gender on secukinumab treatment outcomes, such as disease activity and global functioning and health.
Methods: AQUILA is an ongoing, multi-center, non-interventional study including up to 3000 pts with AS or PsA. Pts were observed from BL up to week (w) 52 according to clinical routine. Real-world data were assessed prospectively and analyzed as observed. Validated questionnaires were used to collect data on disease activity (Bath Ankylosing Spondylitis Disease Activity Index, BASDAI), global functioning and health (Assessment of SpondyloArthritis-Health Index, ASAS-HI) in AS, and skin and joint-related disease activity (Psoriasis Area and Severity Index, PASI; tender/swollen joint counts, TJC/SJC) and impact of disease (Psoriatic Arthritis Impact of Disease - 12 items, PsAID-12 score) in PsA pts. This interim analysis focused on the subgroups of male and female AS and PsA pts stratified by time to diagnosis after disease onset (˂1 year [y] and ≥1y for early and late diagnosis, respectively).
Results: At BL, 609 AS and 1145 PsA pts were included with information on time to diagnosis (Tab. 1); only 18.7% of AS and 25.8% of PsA pts were diagnosed within one year. Of interest, both female AS and PsA pts as well as male PsA pts with increased BMI tended to be diagnosed later (Tab. 1). Regarding BASDAI scores, male AS pts diagnosed late had increased disease activity at BL and throughout the study (Fig. 1A); female AS pts diagnosed late showed reduced total treatment effect with increasing time to diagnosis (Fig. 1B). Similarly, both male and female AS pts diagnosed late had slightly increased ASAS-HI at BL and throughout the study (Tab. 1). For PsA pts, there was no difference in skin- (PASI, Fig. 1C/D) and joint-related (Fig. 1E/F) disease activity with respect to time to diagnosis. Furthermore, there was no difference in PsAID scores (data not shown) between early- and late-diagnosed PsA pts.
Conclusion: In a real-world setting, secukinumab improved disease activity and global functioning in both AS and PsA pts. Both, male and female AS pts had a higher treatment response when diagnosed early. Interestingly, delay in diagnosis appeared to be BMI-dependent in female AS pts and PsA pts of both genders. However, in contrast to AS, treatment response of early- and late-diagnosed PsA pts did not differ in the further course.
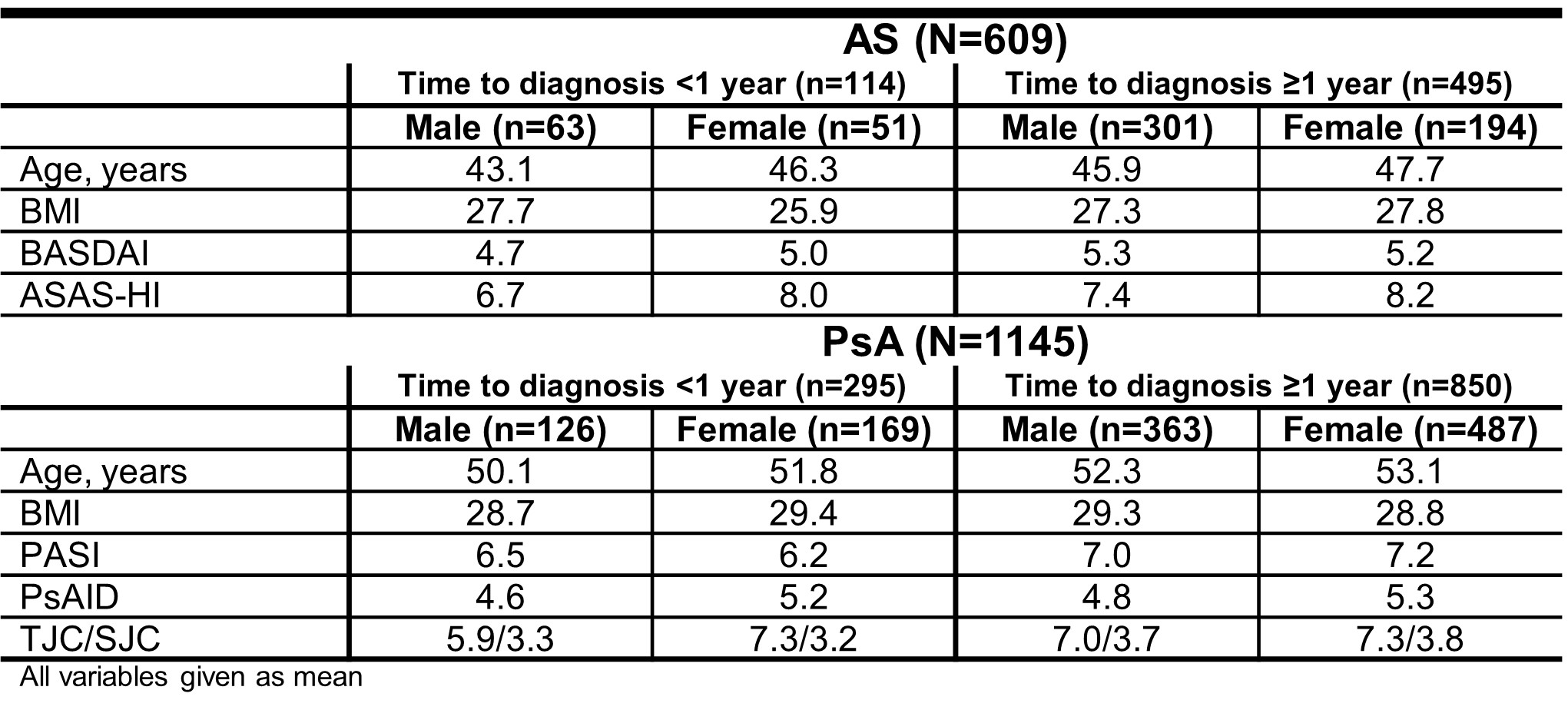 Table 1: Overview of selected BL characteristics in AS and PsA pts stratified by time to diagnosis
Table 1: Overview of selected BL characteristics in AS and PsA pts stratified by time to diagnosis
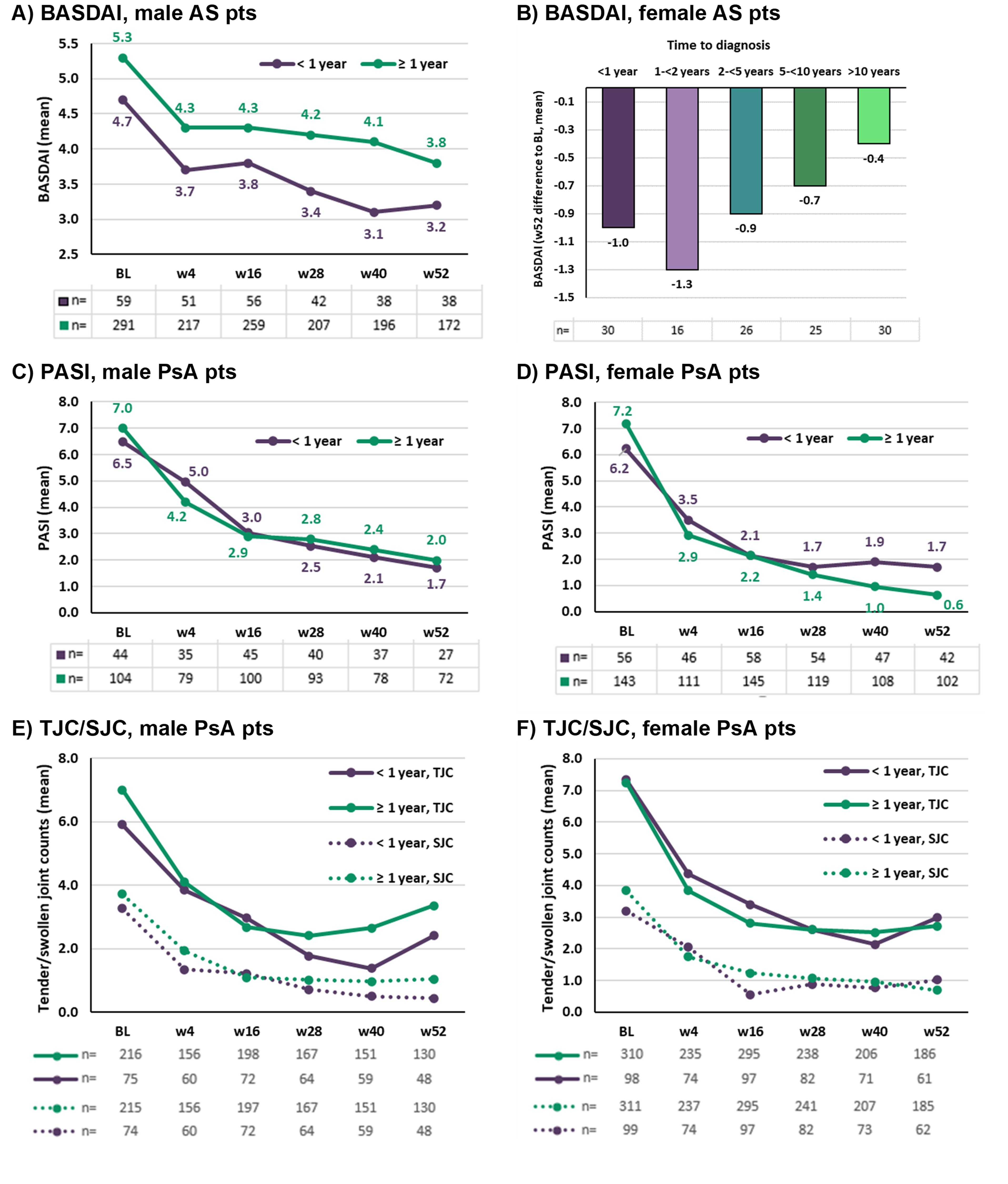 Figure 1: Disease activity in early- and late-diagnosed AS and PsA pts
Figure 1: Disease activity in early- and late-diagnosed AS and PsA pts
Disclosures: K. Benesova, Abbvie, BMS, Gilead/Galapagos, Janssen, Lilly, Medac, MSD, Novartis, Roche, Viatris, Gilead/Galapagos, Novartis, Abbvie, Novartis; O. Hansen, Novartis; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; J. Brandt-Juergens, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, UCB, Sanofi-Aventis, Medac, Gilead, Gilead, Affibody; P. Kästner, Chugai, Novartis; E. Riechers, AbbVie, Chugai, Lilly, Janssen, Novartis, Pfizer, Roche, UCB; D. Peterlik, Novartis; C. Budden, Novartis; A. Boas, Novartis; S. Welle, Novartis; H. Tony, AbbVie, Astra-Zeneca, BMS, Chugai, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi.
Background/Purpose: In both, ankylosing spondylitis (AS) and psoriatic arthritis (PsA), women typically have a longer delay in diagnosis.1,2 There is scientific evidence that prognosis for AS and PsA improves when diagnosed early. The German non-interventional study AQUILA provides real-world data on the influence of time to diagnosis and gender on treatment outcomes under secukinumab, a fully human monoclonal antibody that selectively inhibits interleukin-17A.The aims of this interim analysis are to describe selected baseline (BL) demographics of AS and PsA patients (pts) and to evaluate the impact of time to diagnosis and gender on secukinumab treatment outcomes, such as disease activity and global functioning and health.
Methods: AQUILA is an ongoing, multi-center, non-interventional study including up to 3000 pts with AS or PsA. Pts were observed from BL up to week (w) 52 according to clinical routine. Real-world data were assessed prospectively and analyzed as observed. Validated questionnaires were used to collect data on disease activity (Bath Ankylosing Spondylitis Disease Activity Index, BASDAI), global functioning and health (Assessment of SpondyloArthritis-Health Index, ASAS-HI) in AS, and skin and joint-related disease activity (Psoriasis Area and Severity Index, PASI; tender/swollen joint counts, TJC/SJC) and impact of disease (Psoriatic Arthritis Impact of Disease - 12 items, PsAID-12 score) in PsA pts. This interim analysis focused on the subgroups of male and female AS and PsA pts stratified by time to diagnosis after disease onset (˂1 year [y] and ≥1y for early and late diagnosis, respectively).
Results: At BL, 609 AS and 1145 PsA pts were included with information on time to diagnosis (Tab. 1); only 18.7% of AS and 25.8% of PsA pts were diagnosed within one year. Of interest, both female AS and PsA pts as well as male PsA pts with increased BMI tended to be diagnosed later (Tab. 1). Regarding BASDAI scores, male AS pts diagnosed late had increased disease activity at BL and throughout the study (Fig. 1A); female AS pts diagnosed late showed reduced total treatment effect with increasing time to diagnosis (Fig. 1B). Similarly, both male and female AS pts diagnosed late had slightly increased ASAS-HI at BL and throughout the study (Tab. 1). For PsA pts, there was no difference in skin- (PASI, Fig. 1C/D) and joint-related (Fig. 1E/F) disease activity with respect to time to diagnosis. Furthermore, there was no difference in PsAID scores (data not shown) between early- and late-diagnosed PsA pts.
Conclusion: In a real-world setting, secukinumab improved disease activity and global functioning in both AS and PsA pts. Both, male and female AS pts had a higher treatment response when diagnosed early. Interestingly, delay in diagnosis appeared to be BMI-dependent in female AS pts and PsA pts of both genders. However, in contrast to AS, treatment response of early- and late-diagnosed PsA pts did not differ in the further course.
 Table 1: Overview of selected BL characteristics in AS and PsA pts stratified by time to diagnosis
Table 1: Overview of selected BL characteristics in AS and PsA pts stratified by time to diagnosis Figure 1: Disease activity in early- and late-diagnosed AS and PsA pts
Figure 1: Disease activity in early- and late-diagnosed AS and PsA ptsDisclosures: K. Benesova, Abbvie, BMS, Gilead/Galapagos, Janssen, Lilly, Medac, MSD, Novartis, Roche, Viatris, Gilead/Galapagos, Novartis, Abbvie, Novartis; O. Hansen, Novartis; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; J. Brandt-Juergens, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, UCB, Sanofi-Aventis, Medac, Gilead, Gilead, Affibody; P. Kästner, Chugai, Novartis; E. Riechers, AbbVie, Chugai, Lilly, Janssen, Novartis, Pfizer, Roche, UCB; D. Peterlik, Novartis; C. Budden, Novartis; A. Boas, Novartis; S. Welle, Novartis; H. Tony, AbbVie, Astra-Zeneca, BMS, Chugai, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi.

