Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1166–1185) Systemic Sclerosis and Related Disorders – Basic Science Poster
1177: Identification of a New Type of Pro-phagocytic Macrophages in Patients with Systemic Sclerosis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AH
Amela Hukara, MSc
University Hospital Zurich, University of Zurich
Schlieren, Switzerland
Abstract Poster Presenter(s)
Amela Hukara1, Tracy Tabib2, Michal Rudnik1, Oliver Distler3, Przemyslaw Blyszczuk1, Robert Lafyatis2 and Gabriela Kania1, 1Center of Experimental Rheumatology, Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 2Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, 3Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland
Background/Purpose: Phagocytosis is a crucial cellular process, which under certain immuno-pathological conditions can be activated in macrophages in an uncontrolled manner. Biomarker studies have implicated macrophages in the development and progression of systemic sclerosis (SSc). Fos-like 2 (FOSL-2) transcription factor has been associated with altered macrophages in SSc. We postulate that macrophage phagocytosis is a critical process in SSc progression. The aim of this study was to investigate the role of FOSL-2 in macrophage phagocytosis in patients with SSc.
Methods: Published datasets of single cell RNA sequencing (scRNA-seq) of human explanted lung tissue from SSc-ILD1 and skin from dcSSc patients2 were analyzed using the the R package Seurat V2.3.4. Human monocyte-derived macrophages (hMDM) were differentiated from CD14+ blood-derived monocytes from healthy controls (HC) and SSc patients. hMDM were polarized with LPS (10 ng/ml) or remained unstimulated. Protein expression was detected by Western Blot. pHrodo Red E.coli particles were used to measure phagocytic activity, which was assessed by flow cytometry. hMDM were transfected with FOSL2 or negative control siRNA.
Results: scRNA-seq analyses of the SSc-ILD lung dataset identified differentially expressed phagocytosis-related genes (MARCO, C1QA, C1QB, C1QC) in FOSL2hi SPP1hi lung macrophages from SSc-ILD patients compared to FOSL2null cells (p.adj.≤0.05; log2 ratio≥0.5). Further, scRNA-seq analysis of dcSSc skin tissue revealed that FOSL2hi CCR1+ skin macrophages showed higher expression of phagolysosome-related genes (CORO1A, ARL8B) compared to FOSL2null cells (p.adj.≤0.05; log2 ratio≥0.5). In the in vitro differentiated hMDM, we observed increased FOSL-2 protein levels in unstimulated and pro-inflammatory LPS stimulated SSc hMDM compared to healthy hMDM (Figure 1A). In addition, phagocytic activity of pHrodo Red E.coli particles was increased in unstimulated and LPS stimulated SSc hMDM compared to healthy hMDM (Figure 1B). We did not observe any significant differences in phagocytic activity between early SSc, lcSSc and dcSSc hMDM. To further study the exact role of FOSL2, we used siRNA-specific knockdown of FOSL2 in unstimulated SSc hMDM, which led to reduced phagocytic activity compared to siRNA control hMDM (Figure 1C).
Conclusion: For the first time, we showed that FOSL-2 is a driver of newly identified pro-phagocytic macrophages and a crucial contributor of enhanced macrophage phagocytosis in patients with SSc.
References
1. Valenzi, E., et al., Ann Rheum Dis, 2019, 78(10): p. 1379-1387.
2. Xue, D., et al., Arthritis Rheumatol, 2022, 74(2) : p. 329-341.
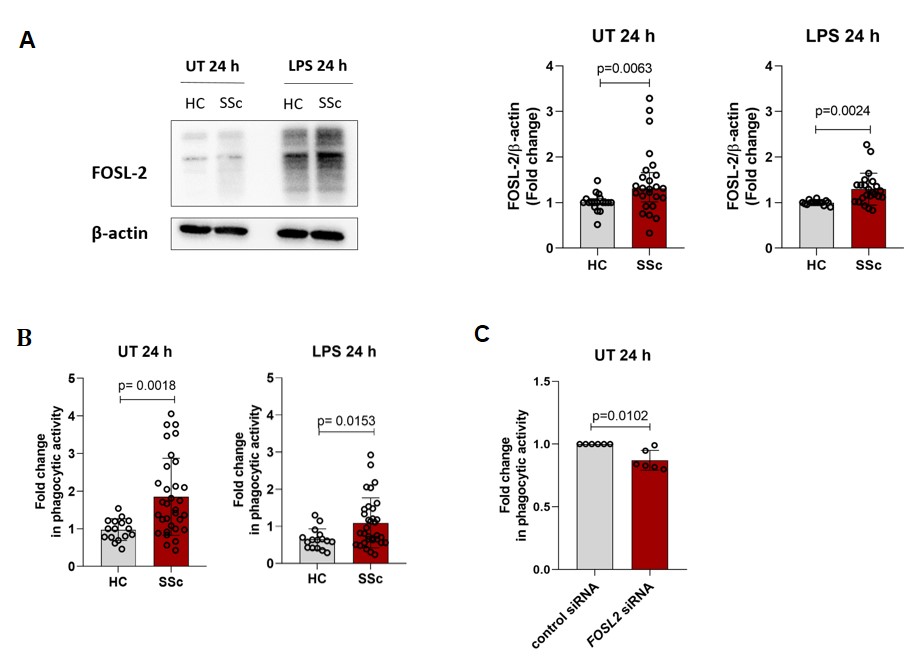 Figure 1: FOSL-2 protein levels and phagocytosis in HC and SSc hMDM. A) Representative Western Blot image and fold changes of FOSL-2 protein levels for unstimulated (UT) and LPS stimulated hMDM, n= HC (11-18), SSc (18-25). B) Fold changes of phagocytic activity are shown for unstimulated (UT) and LPS stimulated hMDM, n= HC (12-16), SSc (29-34). C) Fold changes of phagocytic activity are shown for control siRNA and FOSL2 siRNA silenced unstimulated (UT) hMDM, n= (6).
Figure 1: FOSL-2 protein levels and phagocytosis in HC and SSc hMDM. A) Representative Western Blot image and fold changes of FOSL-2 protein levels for unstimulated (UT) and LPS stimulated hMDM, n= HC (11-18), SSc (18-25). B) Fold changes of phagocytic activity are shown for unstimulated (UT) and LPS stimulated hMDM, n= HC (12-16), SSc (29-34). C) Fold changes of phagocytic activity are shown for control siRNA and FOSL2 siRNA silenced unstimulated (UT) hMDM, n= (6).
Disclosures: A. Hukara, None; T. Tabib, None; M. Rudnik, None; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; P. Blyszczuk, None; R. Lafyatis, Corbus, Formation, Moderna, Regeneron, AstraZeneca, Pfizer, Mitsubishi, Bristol-Myers Squibb(BMS), Boehringer-Ingelheim, Sanofi, Boehringer-Mannheim, Merck, Genentech/Roche, Biogen, Cleveland Clinic, National Institute of Arthritis and Musculoskeletal and Skin Diseases; G. Kania, None.
Background/Purpose: Phagocytosis is a crucial cellular process, which under certain immuno-pathological conditions can be activated in macrophages in an uncontrolled manner. Biomarker studies have implicated macrophages in the development and progression of systemic sclerosis (SSc). Fos-like 2 (FOSL-2) transcription factor has been associated with altered macrophages in SSc. We postulate that macrophage phagocytosis is a critical process in SSc progression. The aim of this study was to investigate the role of FOSL-2 in macrophage phagocytosis in patients with SSc.
Methods: Published datasets of single cell RNA sequencing (scRNA-seq) of human explanted lung tissue from SSc-ILD1 and skin from dcSSc patients2 were analyzed using the the R package Seurat V2.3.4. Human monocyte-derived macrophages (hMDM) were differentiated from CD14+ blood-derived monocytes from healthy controls (HC) and SSc patients. hMDM were polarized with LPS (10 ng/ml) or remained unstimulated. Protein expression was detected by Western Blot. pHrodo Red E.coli particles were used to measure phagocytic activity, which was assessed by flow cytometry. hMDM were transfected with FOSL2 or negative control siRNA.
Results: scRNA-seq analyses of the SSc-ILD lung dataset identified differentially expressed phagocytosis-related genes (MARCO, C1QA, C1QB, C1QC) in FOSL2hi SPP1hi lung macrophages from SSc-ILD patients compared to FOSL2null cells (p.adj.≤0.05; log2 ratio≥0.5). Further, scRNA-seq analysis of dcSSc skin tissue revealed that FOSL2hi CCR1+ skin macrophages showed higher expression of phagolysosome-related genes (CORO1A, ARL8B) compared to FOSL2null cells (p.adj.≤0.05; log2 ratio≥0.5). In the in vitro differentiated hMDM, we observed increased FOSL-2 protein levels in unstimulated and pro-inflammatory LPS stimulated SSc hMDM compared to healthy hMDM (Figure 1A). In addition, phagocytic activity of pHrodo Red E.coli particles was increased in unstimulated and LPS stimulated SSc hMDM compared to healthy hMDM (Figure 1B). We did not observe any significant differences in phagocytic activity between early SSc, lcSSc and dcSSc hMDM. To further study the exact role of FOSL2, we used siRNA-specific knockdown of FOSL2 in unstimulated SSc hMDM, which led to reduced phagocytic activity compared to siRNA control hMDM (Figure 1C).
Conclusion: For the first time, we showed that FOSL-2 is a driver of newly identified pro-phagocytic macrophages and a crucial contributor of enhanced macrophage phagocytosis in patients with SSc.
References
1. Valenzi, E., et al., Ann Rheum Dis, 2019, 78(10): p. 1379-1387.
2. Xue, D., et al., Arthritis Rheumatol, 2022, 74(2) : p. 329-341.
 Figure 1: FOSL-2 protein levels and phagocytosis in HC and SSc hMDM. A) Representative Western Blot image and fold changes of FOSL-2 protein levels for unstimulated (UT) and LPS stimulated hMDM, n= HC (11-18), SSc (18-25). B) Fold changes of phagocytic activity are shown for unstimulated (UT) and LPS stimulated hMDM, n= HC (12-16), SSc (29-34). C) Fold changes of phagocytic activity are shown for control siRNA and FOSL2 siRNA silenced unstimulated (UT) hMDM, n= (6).
Figure 1: FOSL-2 protein levels and phagocytosis in HC and SSc hMDM. A) Representative Western Blot image and fold changes of FOSL-2 protein levels for unstimulated (UT) and LPS stimulated hMDM, n= HC (11-18), SSc (18-25). B) Fold changes of phagocytic activity are shown for unstimulated (UT) and LPS stimulated hMDM, n= HC (12-16), SSc (29-34). C) Fold changes of phagocytic activity are shown for control siRNA and FOSL2 siRNA silenced unstimulated (UT) hMDM, n= (6). Disclosures: A. Hukara, None; T. Tabib, None; M. Rudnik, None; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; P. Blyszczuk, None; R. Lafyatis, Corbus, Formation, Moderna, Regeneron, AstraZeneca, Pfizer, Mitsubishi, Bristol-Myers Squibb(BMS), Boehringer-Ingelheim, Sanofi, Boehringer-Mannheim, Merck, Genentech/Roche, Biogen, Cleveland Clinic, National Institute of Arthritis and Musculoskeletal and Skin Diseases; G. Kania, None.

