Back
Poster Session C
Vasculitis
Session: (1543–1578) Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
1559: Long Term Clinical Effects of Apremilast on Behcet's Disease and Changes in Serum Cytokines
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- YU
Yusuke Ushio, MD
Division of Hematology, Rheumatology and Respiratory Medicine, Department of Internal Medicine, Faculty of Medicine, Kagawa University
Miki, Kita District, Kagawa, Japan
Abstract Poster Presenter(s)
Yusuke Ushio1, Risa Wakiya2, Kiyo Ueeda3, Tomohiro Kameda2, shusaku nakashima4, Hiromi Shimada2, Mikiya Kato2, taichi miyagi5, Rina Mino6, Kanako Chujo7 and Hiroaki Dobashi8, 1Division of Hematology, Rheumatology and Respiratory Medicine, Department of Internal Medicine, Faculty of Medicine, Kagawa University, Miki, Kita District, Kagawa, Japan, 2Kagawa University, Kagawa, Japan, 3Kagawa University, Division of Hematology, Rheumatology and Respiratory Medicine, Department of Internal Medicine, Faculty of Medicine, Kagawa, Japan, 4Division of Hematology, Rheumatology and Respiratory Medicine, Department of Internal Medicine, Faculty of Medicine, Kagawa, Japan, 5Kagawa University, Kidagun, Japan, 6Kagawa University, Division of Hematology, Rheumatology and Respiratory Medicine, Department of Internal Medicine, Faculty of Medicine, Miki-cho, Kita-gun, Japan, 7Kagawa University, Miki, Kita District, Kagawa, Japan, 8Division of Hematology, Rheumatology and Respiratory Medicine, Department of Internal Medicine, Faculty of Medicine, Kagawa, Kagawa, Japan
Background/Purpose: Apremilast, the small-molecule phosphodiesterase -4 inhibitor, was approved for the treatment of recurrent oral ulcers associated with Behcet's disease (BD) in Japan from September 2019. However, the efficacy of apremilast for long-term treatment beyond 1 year is unknown. In addition, it has been reported that apremilast may decrease the production of inflammatory cytokines and increase the production of anti-inflammatory mediators in psoriasis and psoriatic arthritis, whereas the regulation of cytokines in BD is fully clarified. Therefore, we aimed to investigate the effects of apremilast on multiple lesion domains including oral ulcers and blood cytokine levels in BD.
Methods: Patients with BD who were started on apremilast at our hospital were included in the study. We analyzed the clinical effects of apremilast on oral ulcers, genital ulcers, skin lesions, arthritis, and arthralgia until 18 months after treatment. Changes in serum cytokines (IFN-γ, TNF-α, IL-6, IL-8, IL-10, IL-23, MIP-1β, MCP-1, and IL-1RA) were measured before and 3 months after treatment using the multiplex Luminex immunoassay (R&D Systems) and Simple Plex (ProteinSimple, CA, USA). The relationship between the clinical effects and the changes in cytokines was analyzed.
Results: Sixteen patients (4 males, 12 females) were included in the study (Table 1). The mean age was 46.6±12.2 years, and the mean disease duration was 10.6±8.3 years. The organ manifestations observed at the start of apremilast treatment included oral ulcers(100%), genital ulcers (31.3%), skin lesions (62.5%), arthritis (37.5%) and arthralgia (62.5%). Clinical effects of apremilast on each lesion up to 18 months are shown in Figure 1. At 6, 12, and 18 months after starting apremilast, 93.3%, 92.3%, and 66.7% of patients had resolution of oral ulcers, respectively. Genital ulcers resolved in all patients after 6 months and did not relapse in the following period. As for skin and joint lesions, at 12 months, skin lesions were observed in 7 of 13 patients (53.8%), arthritis in 3 of 13 patients (23.0%), and arthralgia in 6 of 13 patients (46.1%). Furthermore, at 18 months, skin lesions were observed in 2 of 6 patients (33.3%), arthritis in 3 of 6 patients (50.0%), and arthralgia in 4 of 6 patients (66.7%). Thus, for joint lesions, there was a trend toward worsening from 12 to 18 months. In the seven patients for whom serum cytokines could be analyzed, serum TNF-α, IL-23, and MIP-1β levels were decreased 3 months after apremilast treatment. Serum IL-6, IL-10, IFN-γ, and MIP-1β levels were more decreased in the group of patients whose oral ulcers disappeared after 3 months compared to the group of patients whose oral ulcers remained. Furthermore, serum MCP-1 level was decreased in patients with skin lesions, and serum IL-8 level was decreased in the patients with arthritis.
Conclusion: The long-term efficacy of apremilast differs between oral and genital ulcers, skin lesions and joint lesions, suggesting that there may be differences in the long-term efficacy of apremilast in each manifestation. Cytokine changes after apremilast administration in BD suggest that they may be related to the type of lesion and therapeutic response.
.jpg) Table 1. Baseline characteristics of patients with Behçet’s disease enrolled in the study. BD, Behçet’s disease; SD, standard deviation
Table 1. Baseline characteristics of patients with Behçet’s disease enrolled in the study. BD, Behçet’s disease; SD, standard deviation
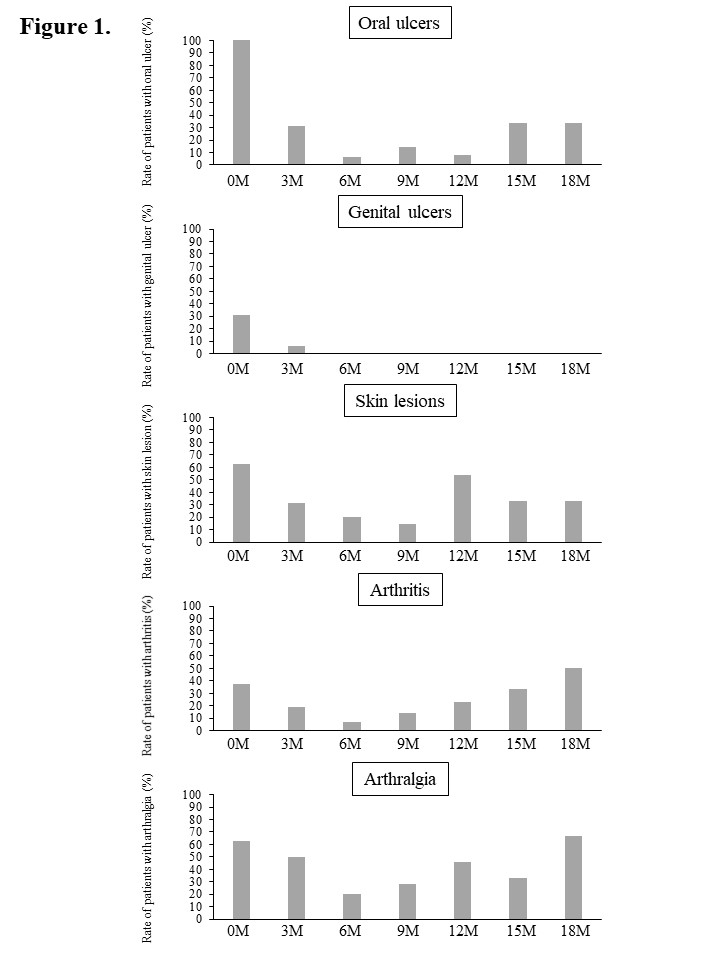 Figure 1. Clinical effects of apremilast on each lesion up to 18 months.
Figure 1. Clinical effects of apremilast on each lesion up to 18 months.
Disclosures: Y. Ushio, None; R. Wakiya, None; K. Ueeda, None; T. Kameda, None; s. nakashima, None; H. Shimada, None; M. Kato, None; t. miyagi, None; R. Mino, None; K. Chujo, None; H. Dobashi, Bristol-Myers Squibb (BMS), Chugai, Eli Lilly, GlaxoSmithKlein (GSK), MSD, Novartis, Pfizer, UCB Pharma.
Background/Purpose: Apremilast, the small-molecule phosphodiesterase -4 inhibitor, was approved for the treatment of recurrent oral ulcers associated with Behcet's disease (BD) in Japan from September 2019. However, the efficacy of apremilast for long-term treatment beyond 1 year is unknown. In addition, it has been reported that apremilast may decrease the production of inflammatory cytokines and increase the production of anti-inflammatory mediators in psoriasis and psoriatic arthritis, whereas the regulation of cytokines in BD is fully clarified. Therefore, we aimed to investigate the effects of apremilast on multiple lesion domains including oral ulcers and blood cytokine levels in BD.
Methods: Patients with BD who were started on apremilast at our hospital were included in the study. We analyzed the clinical effects of apremilast on oral ulcers, genital ulcers, skin lesions, arthritis, and arthralgia until 18 months after treatment. Changes in serum cytokines (IFN-γ, TNF-α, IL-6, IL-8, IL-10, IL-23, MIP-1β, MCP-1, and IL-1RA) were measured before and 3 months after treatment using the multiplex Luminex immunoassay (R&D Systems) and Simple Plex (ProteinSimple, CA, USA). The relationship between the clinical effects and the changes in cytokines was analyzed.
Results: Sixteen patients (4 males, 12 females) were included in the study (Table 1). The mean age was 46.6±12.2 years, and the mean disease duration was 10.6±8.3 years. The organ manifestations observed at the start of apremilast treatment included oral ulcers(100%), genital ulcers (31.3%), skin lesions (62.5%), arthritis (37.5%) and arthralgia (62.5%). Clinical effects of apremilast on each lesion up to 18 months are shown in Figure 1. At 6, 12, and 18 months after starting apremilast, 93.3%, 92.3%, and 66.7% of patients had resolution of oral ulcers, respectively. Genital ulcers resolved in all patients after 6 months and did not relapse in the following period. As for skin and joint lesions, at 12 months, skin lesions were observed in 7 of 13 patients (53.8%), arthritis in 3 of 13 patients (23.0%), and arthralgia in 6 of 13 patients (46.1%). Furthermore, at 18 months, skin lesions were observed in 2 of 6 patients (33.3%), arthritis in 3 of 6 patients (50.0%), and arthralgia in 4 of 6 patients (66.7%). Thus, for joint lesions, there was a trend toward worsening from 12 to 18 months. In the seven patients for whom serum cytokines could be analyzed, serum TNF-α, IL-23, and MIP-1β levels were decreased 3 months after apremilast treatment. Serum IL-6, IL-10, IFN-γ, and MIP-1β levels were more decreased in the group of patients whose oral ulcers disappeared after 3 months compared to the group of patients whose oral ulcers remained. Furthermore, serum MCP-1 level was decreased in patients with skin lesions, and serum IL-8 level was decreased in the patients with arthritis.
Conclusion: The long-term efficacy of apremilast differs between oral and genital ulcers, skin lesions and joint lesions, suggesting that there may be differences in the long-term efficacy of apremilast in each manifestation. Cytokine changes after apremilast administration in BD suggest that they may be related to the type of lesion and therapeutic response.
.jpg) Table 1. Baseline characteristics of patients with Behçet’s disease enrolled in the study. BD, Behçet’s disease; SD, standard deviation
Table 1. Baseline characteristics of patients with Behçet’s disease enrolled in the study. BD, Behçet’s disease; SD, standard deviation Figure 1. Clinical effects of apremilast on each lesion up to 18 months.
Figure 1. Clinical effects of apremilast on each lesion up to 18 months.Disclosures: Y. Ushio, None; R. Wakiya, None; K. Ueeda, None; T. Kameda, None; s. nakashima, None; H. Shimada, None; M. Kato, None; t. miyagi, None; R. Mino, None; K. Chujo, None; H. Dobashi, Bristol-Myers Squibb (BMS), Chugai, Eli Lilly, GlaxoSmithKlein (GSK), MSD, Novartis, Pfizer, UCB Pharma.

