Back
Poster Session C
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: (1360–1386) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
1377: Mental Health Screening in Juvenile Myositis: Preliminary Analysis of a Multicenter Pilot Study
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- KA
Kaveh Ardalan, MD, MS
Duke University Medical Center
Durham, NC, United States
Abstract Poster Presenter(s)
Kaveh Ardalan1, Lindsay Olson1, Jeffrey Dvergsten2, Ann Reed1, Alison Manning1, Gary Maslow1, Aruna Rikhi1, Brian Feldman3, ashley Danguecan4, Sarah Mossad4, Luana Flores Pereira4, Susan Shenoi5, Stacey Haynes5, Joanna Patten5 and Andrea Knight6, 1Duke University School of Medicine, Durham, NC, 2Duke University Hospital, Durham, NC, 3Division of Rheumatology, The Hospital for Sick Children; Child Health Evaluative Services, SickKids Research Institute; Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 4The Hospital for Sick Children, Toronto, ON, Canada, 5Seattle Children's Hospital and Research Center / University of Washington, Seattle, WA, 6The Hospital for Sick Children, Division of Rheumatology, Department of Paediatrics, University of Toronto, Toronto, ON, Canada
Background/Purpose: Qualitative studies in juvenile myositis (JM) suggest high rates of emotional distress but the prevalence of mental health comorbidities is not well described. We present the first multicenter North American study assessing acceptability of mental health screening, rates of positive mental health screening results, and associations of mental health with outcomes/health behaviors in JM.
Methods: JM patients/parents (5-21 yo) were eligible. Patient/parent-proxy mental health screeners included: Pediatric Symptom Checklist-17 (PSC-17), Patient Health Questionnaire-9 (PHQ9), and Screen for Child Anxiety Related Disorders (SCARED). Positive screening was defined as any total/domain score above established cutoffs. Patients/parents completed a 3-item mental health screening acceptability survey. Patient-Reported Outcomes Measurement Information System (PROMIS) pediatric/parent-proxy Depressive Symptoms, Anxiety, Psychological Stress Experiences, and Positive Affect measures assessed emotional distress intensity. Outcomes/health behavior measures included: 1) Physician/Patient/Parent-proxy Global Assessments of Disease Activity (PGA, Pt/Par-GA); 2) PROMIS Mobility/Upper Extremity Function; 3) PROMIS Physical Activity; 4) medication adherence (Domains of Subjective Extent of Nonadherence [DOSE-NA]); 5) Measures of Sun Protection Practices. Mann-Whitney U test assessed positive vs negative screening group differences in the outcomes/health behavior measures. Spearman's correlations for PROMIS measures of emotional distress and the outcomes/health behaviors were calculated.
Results: Data from 72 participants were analyzed. Most patients had dermatomyositis ( >90%) (Table 1). Most patients/parents respectively rated mental health screeners 'a little/not at all' difficult to complete (94.3%; 97%), and agreed screening should continue (94.3%; 94%) at least annually (88.6%; 95.5%). The majority screened positive on at least one mental health screening measure (n=51; 71%) (Table 2). Of patients who completed the PHQ-9, 20% scored ≥ 10, consistent with at least moderate depression symptoms. Positive screening was associated with higher PROMIS Depressive Symptoms, Anxiety, and Psychological Stress scores by patient (all p < 0.001) and parent-proxy report (p = 0.006, 0.003, 0.01 respectively) and lower patient PROMIS Positive Affect score (p = 0.025). Positive screening was not significantly associated with differences in outcomes/health behaviors. Correlational analysis showed associations between worse scores for PROMIS emotional distress measures and poorer physical function, worse medication nonadherence, and higher Par-GA score, though correlation coefficients were small in magnitude (Table 3).
Conclusion: JM patients/parents find routine mental health screening acceptable and necessary. High rates of positive screening suggest substantial psychosocial burden and worse mental health may correlate with poorer health outcomes/behaviors. Future studies should assess mental health longitudinally, evaluate mental health referral patterns, and further assess relationships to outcomes.
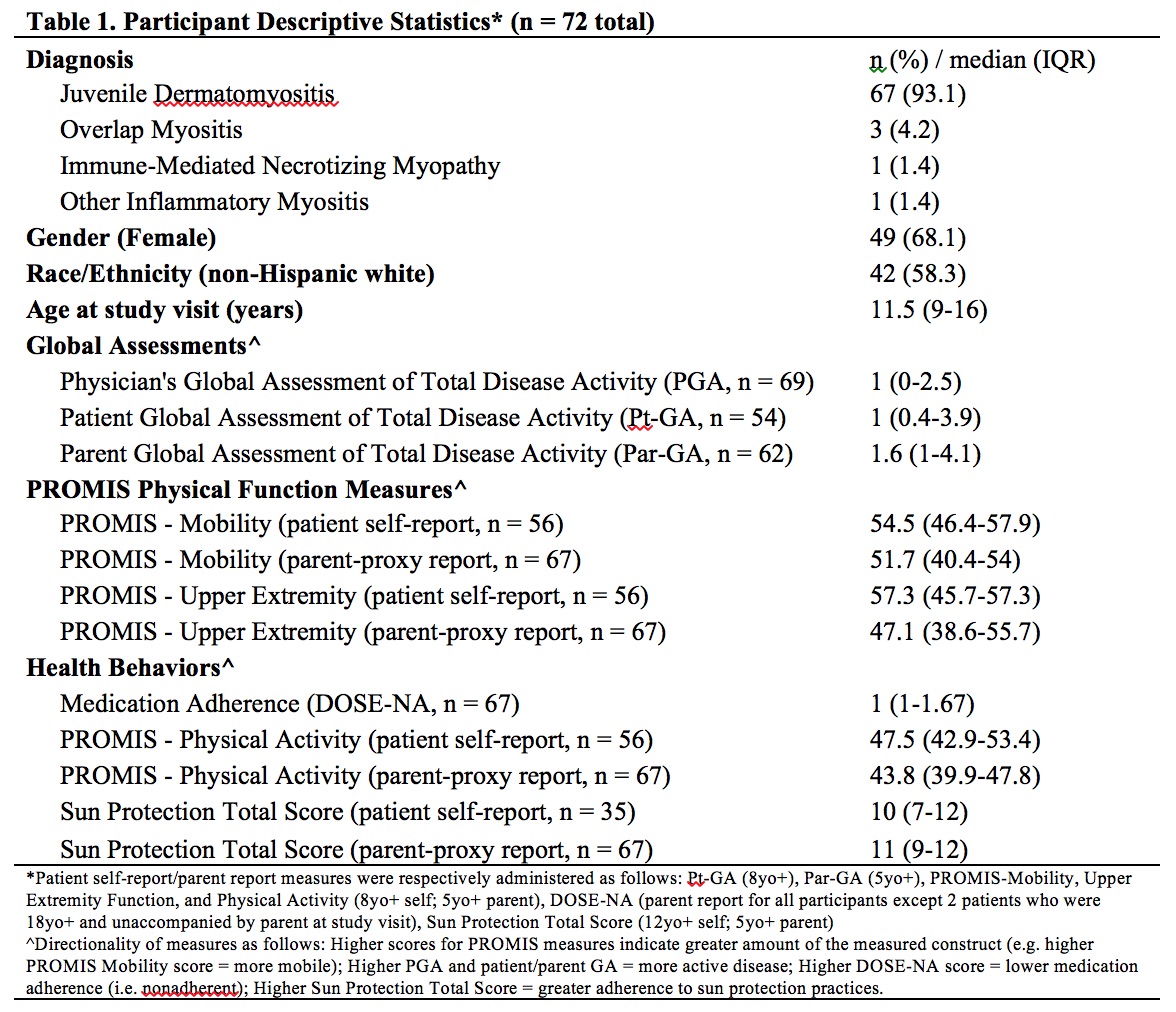 Table 1: Participant Descriptive Statistics
Table 1: Participant Descriptive Statistics
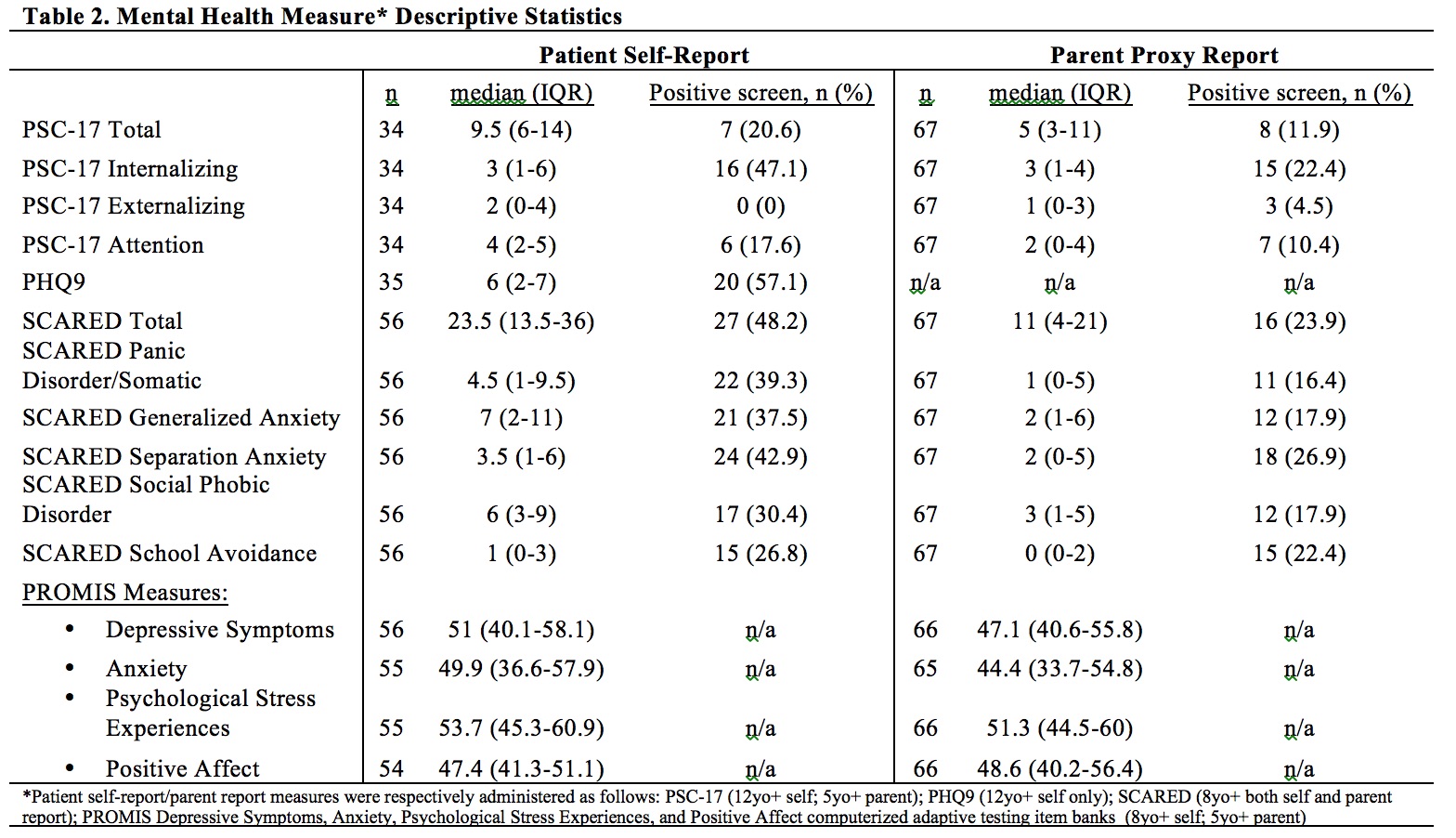 Table 2: Mental Health Measure Descriptive Statistics
Table 2: Mental Health Measure Descriptive Statistics
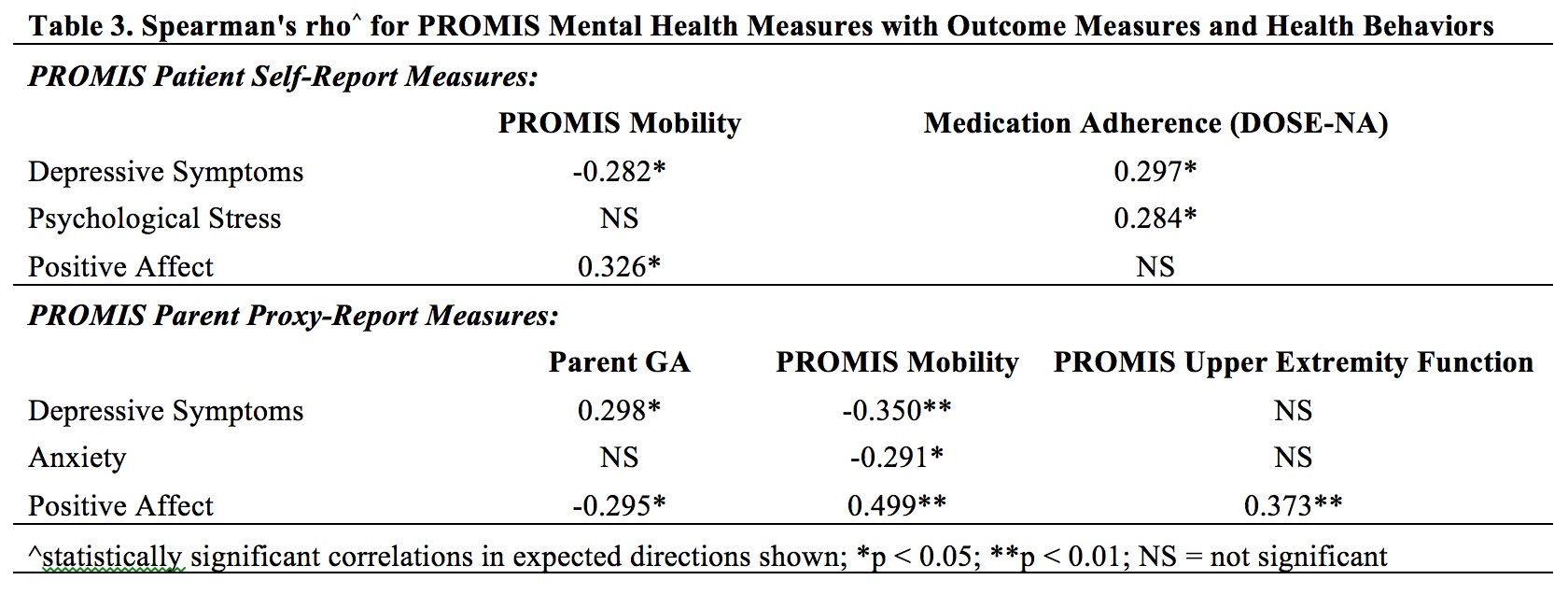 Table 3: Spearman's rho for PROMIS Mental Health Measures with Outcome Measures and Health Behaviors
Table 3: Spearman's rho for PROMIS Mental Health Measures with Outcome Measures and Health Behaviors
Disclosures: K. Ardalan, None; L. Olson, None; J. Dvergsten, None; A. Reed, None; A. Manning, None; G. Maslow, None; A. Rikhi, None; B. Feldman, Pfizer, AB2-Bio, Janssen; a. Danguecan, None; S. Mossad, None; L. Flores Pereira, None; S. Shenoi, None; S. Haynes, None; J. Patten, None; A. Knight, None.
Background/Purpose: Qualitative studies in juvenile myositis (JM) suggest high rates of emotional distress but the prevalence of mental health comorbidities is not well described. We present the first multicenter North American study assessing acceptability of mental health screening, rates of positive mental health screening results, and associations of mental health with outcomes/health behaviors in JM.
Methods: JM patients/parents (5-21 yo) were eligible. Patient/parent-proxy mental health screeners included: Pediatric Symptom Checklist-17 (PSC-17), Patient Health Questionnaire-9 (PHQ9), and Screen for Child Anxiety Related Disorders (SCARED). Positive screening was defined as any total/domain score above established cutoffs. Patients/parents completed a 3-item mental health screening acceptability survey. Patient-Reported Outcomes Measurement Information System (PROMIS) pediatric/parent-proxy Depressive Symptoms, Anxiety, Psychological Stress Experiences, and Positive Affect measures assessed emotional distress intensity. Outcomes/health behavior measures included: 1) Physician/Patient/Parent-proxy Global Assessments of Disease Activity (PGA, Pt/Par-GA); 2) PROMIS Mobility/Upper Extremity Function; 3) PROMIS Physical Activity; 4) medication adherence (Domains of Subjective Extent of Nonadherence [DOSE-NA]); 5) Measures of Sun Protection Practices. Mann-Whitney U test assessed positive vs negative screening group differences in the outcomes/health behavior measures. Spearman's correlations for PROMIS measures of emotional distress and the outcomes/health behaviors were calculated.
Results: Data from 72 participants were analyzed. Most patients had dermatomyositis ( >90%) (Table 1). Most patients/parents respectively rated mental health screeners 'a little/not at all' difficult to complete (94.3%; 97%), and agreed screening should continue (94.3%; 94%) at least annually (88.6%; 95.5%). The majority screened positive on at least one mental health screening measure (n=51; 71%) (Table 2). Of patients who completed the PHQ-9, 20% scored ≥ 10, consistent with at least moderate depression symptoms. Positive screening was associated with higher PROMIS Depressive Symptoms, Anxiety, and Psychological Stress scores by patient (all p < 0.001) and parent-proxy report (p = 0.006, 0.003, 0.01 respectively) and lower patient PROMIS Positive Affect score (p = 0.025). Positive screening was not significantly associated with differences in outcomes/health behaviors. Correlational analysis showed associations between worse scores for PROMIS emotional distress measures and poorer physical function, worse medication nonadherence, and higher Par-GA score, though correlation coefficients were small in magnitude (Table 3).
Conclusion: JM patients/parents find routine mental health screening acceptable and necessary. High rates of positive screening suggest substantial psychosocial burden and worse mental health may correlate with poorer health outcomes/behaviors. Future studies should assess mental health longitudinally, evaluate mental health referral patterns, and further assess relationships to outcomes.
 Table 1: Participant Descriptive Statistics
Table 1: Participant Descriptive Statistics Table 2: Mental Health Measure Descriptive Statistics
Table 2: Mental Health Measure Descriptive Statistics Table 3: Spearman's rho for PROMIS Mental Health Measures with Outcome Measures and Health Behaviors
Table 3: Spearman's rho for PROMIS Mental Health Measures with Outcome Measures and Health BehaviorsDisclosures: K. Ardalan, None; L. Olson, None; J. Dvergsten, None; A. Reed, None; A. Manning, None; G. Maslow, None; A. Rikhi, None; B. Feldman, Pfizer, AB2-Bio, Janssen; a. Danguecan, None; S. Mossad, None; L. Flores Pereira, None; S. Shenoi, None; S. Haynes, None; J. Patten, None; A. Knight, None.

